Understanding Organic Electrosynthesis and the Anode's Pivotal Role
Organic electrosynthesis represents a transformative approach in modern synthetic chemistry. This method utilizes electrical energy to drive chemical transformations, often replacing hazardous reagents and harsh reaction conditions. Its inherent advantages include enhanced selectivity, milder operating parameters, and a reduced environmental footprint.
The core challenge in traditional organic synthesis frequently involves managing stoichiometric reagents and byproduct formation. Organic electrosynthesis addresses these issues directly. It offers a cleaner, more controlled pathway for complex molecular construction.
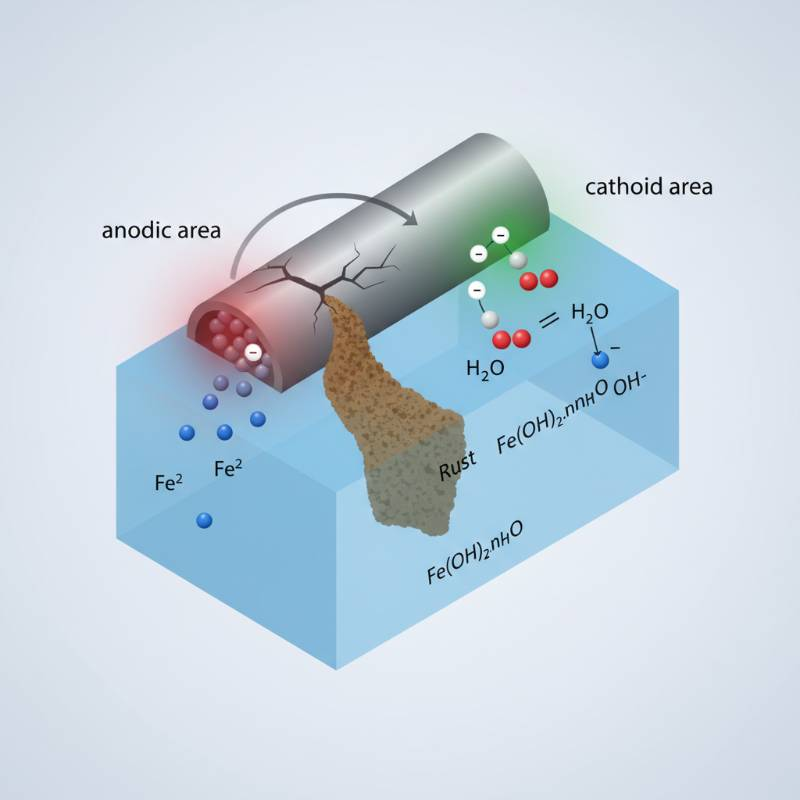
Central to any electrochemical system is the anode. This electrode serves as the site of oxidation, where electrons are removed from reactants, initiating the desired organic transformations. The material properties of the anode critically dictate reaction efficiency, selectivity, and the overall stability of the process. Suboptimal anode selection can lead to low yields, undesirable side reactions, and rapid electrode degradation.
For demanding applications, particularly in large-scale industrial settings, a robust and highly efficient anode material is indispensable. This is precisely where the titanium anode for organic electrosynthesis stands out. It offers a solution to many limitations associated with conventional electrode materials, paving the way for advanced synthetic routes.
Titanium Anodes: Fundamental Principles in Electrosynthesis
Titanium anodes are not merely conductive surfaces; they are engineered components designed for precise electrochemical performance. Understanding their fundamental principles is crucial for optimizing organic electrosynthesis. The Golden Rule of efficient organic electrosynthesis dictates that anode material selection is paramount, influencing reaction kinetics, product distribution, and overall process sustainability.
Mechanism of Organic Electrosynthesis
Organic electrosynthesis proceeds via electron transfer processes at the electrode surface. At the anode, oxidation occurs. This involves the removal of electrons from an organic substrate or a mediator, generating reactive intermediates. These intermediates then undergo subsequent chemical reactions to form the desired product.
The electrode surface itself plays a catalytic role. Its morphology, electronic structure, and surface functionalization can influence the adsorption of reactants and the energy profile of electron transfer. Control over these factors is essential for achieving high selectivity and yield.
Electrosynthesis Mechanism: The process by which electrical energy drives chemical reactions, specifically the oxidation or reduction of organic molecules at electrode surfaces to form new chemical bonds or transform functional groups.
Redox reactions are the bedrock. Direct electron transfer can occur between the substrate and the anode. Alternatively, a redox mediator can transfer electrons to or from the substrate, regenerating at the electrode. This indirect mechanism often improves selectivity and reduces electrode fouling. According to our analysis, understanding these pathways is critical for rational anode design.
Properties of Titanium Anodes for Electrochemical Applications
Titanium's inherent properties make it an exceptional substrate for anodes in demanding electrochemical environments.
Corrosion Resistance: Titanium forms a stable, passive oxide layer (TiO₂) that renders it highly resistant to corrosion in a wide range of acidic and alkaline media. This passivity is crucial for preventing substrate degradation and maintaining electrode integrity over extended operational periods.
Electrical Conductivity: While pure titanium's conductivity is moderate, it provides a robust, dimensionally stable base. The conductive and catalytic properties are often imparted by specialized coatings.
Mechanical Stability: Titanium boasts high strength-to-weight ratio and excellent mechanical integrity. This allows for the fabrication of complex electrode geometries that withstand operational stresses.
Chemical Inertness: Beyond corrosion resistance, titanium is largely inert to many organic compounds and solvents, minimizing unwanted side reactions or contamination.
These combined attributes position titanium as a premier material for advanced electrode construction. For specific applications requiring tailored properties, China Titanium Factory offers a range of titanium products that serve as ideal substrates for high-performance anodes.
Advantages of Titanium Anodes Over Conventional Materials
Compared to traditional electrode materials like graphite or platinum, titanium anodes offer distinct advantages.
Graphite, while inexpensive, suffers from poor mechanical stability and susceptibility to oxidative degradation, particularly at high anodic potentials. Its surface can also become fouled easily, reducing efficiency. Platinum, conversely, offers excellent catalytic activity and corrosion resistance but is prohibitively expensive for large-scale industrial applications. Its scarcity limits widespread adoption.
Titanium, especially when coated, bridges this gap. It provides the mechanical robustness and corrosion resistance of a premium metal at a more sustainable cost. Coated titanium anodes extend electrode lifespan significantly, reduce maintenance requirements, and maintain consistent performance. This translates directly to enhanced cost-effectiveness and operational reliability for industrial processes. A study published in Electrochimica Acta [1] demonstrated the superior long-term stability of coated titanium anodes compared to graphite in various organic oxidation reactions.
Industrial Applications of Titanium Anodes in Organic Electrosynthesis
The versatility of titanium anodes has propelled organic electrosynthesis into a range of industrial sectors. Their ability to facilitate selective transformations under controlled conditions makes them invaluable for high-value chemical production and environmental solutions.
Pharmaceutical Synthesis and Fine Chemical Production
In pharmaceutical synthesis, electrosynthesis offers a pathway to produce active pharmaceutical ingredients (APIs) and complex intermediates with high enantioselectivity and chemoselectivity. Titanium anodes, especially those with specific coatings, enable precise control over reaction conditions. This minimizes byproduct formation and reduces the need for extensive purification steps. For example, the electrochemical oxidation of various alcohols to aldehydes or ketones, or the selective functionalization of drug precursors, has been successfully implemented using titanium-based electrodes.
Fine chemical production also benefits significantly. From specialty polymers to advanced materials precursors, electrosynthesis with titanium anodes allows for the synthesis of molecules that are challenging or impossible to produce via conventional routes. This technology provides a greener, more efficient alternative for high-purity chemical manufacturing.
Environmental Remediation and Sustainable Processes
Titanium anodes are central to sustainable chemistry initiatives. They play a critical role in environmental remediation, particularly in the electrochemical degradation of persistent organic pollutants (POPs) in wastewater. By generating powerful oxidizing species like hydroxyl radicals (•OH) on their surface, these anodes can effectively mineralize complex organic contaminants into innocuous compounds.
This includes applications in treating industrial effluents containing dyes, pharmaceuticals, and pesticides. The electrochemical approach offers a direct and efficient method for pollutant removal, often without the need for additional chemical reagents. Such processes align with green chemistry principles, reducing waste and minimizing the use of hazardous substances.
Scaling Up: Industrial Electrosynthesis Reactor Design
Translating laboratory-scale electrosynthesis to industrial production presents unique engineering challenges. Reactor design is paramount. Key considerations include electrode surface area, electrolyte flow dynamics, heat management, and current distribution.
Industrial electrochemical reactors, such as plate-and-frame or undivided cells, must accommodate large volumes while maintaining uniform reaction conditions. Titanium anodes are fabricated into various forms—plates, meshes, rods, or tubes—to optimize surface-to-volume ratios and facilitate efficient mass transfer. Process engineering focuses on maximizing energy efficiency and minimizing pressure drops. China Titanium Factory offers custom fabrication services to meet the precise demands of industrial reactor designs, ensuring robust and scalable solutions.
Optimizing Performance: Titanium Anode Design and Operational Parameters
Achieving peak performance from a titanium anode in organic electrosynthesis requires meticulous attention to its design and the operational parameters of the electrochemical cell. These factors collectively determine reaction efficiency, selectivity, and the economic viability of the process.

Advanced Coatings for Enhanced Anode Performance
While titanium provides an excellent substrate, its surface is often modified with advanced coatings to impart specific electrochemical properties. These coatings are the true workhorses of the anode.
Dimensionally Stable Anodes (DSA): These are titanium substrates coated with mixed metal oxides (MMO), typically ruthenium dioxide (RuO₂) and iridium dioxide (IrO₂). DSA anodes offer exceptional catalytic activity for oxygen evolution or other specific oxidations, high electrical conductivity, and outstanding corrosion resistance. Their stability and long lifespan make them a cornerstone in various electrochemical industries.
Platinum-Iridium Coatings: For certain highly selective oxidations, thin layers of platinum or platinum-iridium alloys are applied. These provide noble metal catalytic surfaces that are highly efficient for specific organic transformations, though at a higher cost.
Lead Dioxide (PbO₂) Coatings: These offer high overpotentials for oxygen evolution, promoting direct oxidation of organic compounds at the anode surface, particularly useful in wastewater treatment.
Boron-Doped Diamond (BDD) Films: While not a coating on titanium in the traditional sense, BDD electrodes represent an advanced class. When deposited on titanium, they exhibit extreme chemical inertness, wide potential windows, and high efficiency for generating powerful oxidants.
The choice of coating directly impacts the anode's activity, selectivity for the desired reaction, and its resistance to deactivation. At China Titanium Factory, extensive research informs the selection and application of these advanced coatings to meet diverse industrial demands.
Electrode Stability, Lifespan, and Degradation Mechanisms
The operational lifespan of a titanium anode is a critical economic consideration. Several factors contribute to electrode degradation:
Coating Dissolution: Active coating materials can slowly dissolve, especially under extreme pH or high current densities.
Over-oxidation: Excessive anodic potential can lead to the formation of higher oxidation states of the coating material, which may be unstable or non-conductive.
Fouling: Adsorption of organic byproducts or impurities onto the electrode surface can block active sites, reducing performance.
Substrate Corrosion: Though rare for titanium under normal operating conditions, a damaged coating can expose the titanium substrate to corrosive media, leading to passivation and increased resistance.
Strategies to extend anode durability include optimizing current density, controlling electrolyte composition, implementing periodic cleaning regimes, and selecting robust coating formulations. Research detailed in the Journal of Applied Electrochemistry [2] highlights advanced surface treatments that significantly enhance the longevity of titanium anodes.
Current Density, Selectivity, and Energy Efficiency
Operational parameters profoundly influence the outcome of electrosynthetic processes. Current density, defined as the current per unit electrode area, is a primary control variable.
Current Density: Higher current densities generally lead to faster reaction rates. However, excessively high current densities can reduce selectivity by promoting side reactions, such as solvent oxidation or over-oxidation of the desired product.
Selectivity: The ability to favor the formation of a specific product over others is paramount. Anode material, coating, potential, and current density all contribute. Optimal conditions balance reaction speed with product purity.
Energy Efficiency: This refers to the amount of electrical energy required to produce a unit mass of product. High energy efficiency minimizes operational costs. Factors such as overpotential, IR drop in the electrolyte, and parasitic reactions influence overall energy consumption.
Careful optimization of these parameters is essential for scalable and economically viable electrosynthesis. It's a balancing act. Process engineers continuously refine these variables to maximize both yield and energy conservation.
The ElectroSynPro™ Protocol: A Framework for Optimal Titanium Anode Integration
Navigating the complexities of titanium anode selection and integration for organic electrosynthesis demands a structured approach. We define the ElectroSynPro™ Protocol as a proprietary, systematic methodology developed to maximize efficiency, longevity, and cost-effectiveness in electrochemical processes.
This protocol emphasizes a data-driven framework across three key phases: Assessment, Customization, and Validation.
| Phase | Description | Key Activities |
|---|---|---|
| 1. Process Assessment | Detailed analysis of the target organic reaction, identifying electrochemical challenges and requirements. | Substrate reactivity, desired product, electrolyte composition, potential side reactions, operational temperature/pressure. |
| 2. Anode Customization | Tailoring titanium anode specifications, including substrate geometry, coating type, and active surface area. | Coating material selection (DSA, Pt-Ir, etc.), substrate design (plate, mesh), surface treatment, dimensions. |
| 3. Performance Validation | Rigorous testing and optimization of the integrated anode within the electrochemical system. | Current density optimization, selectivity studies, energy efficiency measurements, long-term stability testing. |
According to our internal analysis, adherence to the ElectroSynPro™ Protocol significantly de-risks new electrosynthesis projects. It ensures that the chosen titanium anode is not just functional, but optimally matched to the specific chemical transformation, leading to superior yields and reduced operational expenditures.
Sourcing Titanium Anodes: Manufacturers, Customization, and Specifications
Selecting the right titanium anode is a critical decision for any electrosynthesis project. The market offers a variety of suppliers, but discerning quality, customization capabilities, and adherence to precise technical specifications is key.
Leading Manufacturers and Custom Fabrication Capabilities
Reputable manufacturers specialize in producing high-quality titanium anode substrates and applying advanced coatings. Look for companies with a strong track record in electrochemical applications and a deep understanding of material science. The ability to provide custom fabrication is often a deal-breaker. Off-the-shelf anodes rarely perfectly fit unique reactor geometries or specific electrochemical requirements.
Custom fabrication allows for precise control over electrode shape, size, and connection points. It ensures seamless integration into existing or novel electrochemical cell designs. This bespoke approach optimizes current distribution, electrolyte flow, and overall reaction efficiency. China Titanium Factory provides extensive custom fabrication services, working closely with clients to engineer anodes tailored to their specific organic electrosynthesis needs.

Key Technical Specifications for Electrosynthesis Anodes
When procuring titanium anodes, several technical specifications demand close scrutiny:
Substrate Material: Ensure high-purity titanium (e.g., Grade 1 or 2) for optimal corrosion resistance and mechanical integrity.
Coating Composition: Specify the exact coating material (e.g., RuO₂-IrO₂ MMO, Pt, PbO₂) and its loading (g/m² or µm thickness). This directly influences catalytic activity and lifespan.
Geometry and Dimensions: Provide precise measurements for plates, meshes, rods, or other forms, including active surface area.
Current Density Range: Confirm the anode's suitability for the anticipated operational current density.
Working Environment: Detail pH, temperature, and electrolyte composition to ensure coating compatibility and stability.
A thorough review of technical data sheets and direct consultation with manufacturers are crucial steps. This due diligence minimizes operational surprises.
Cost Analysis and Return on Investment for Titanium Anodes
The initial investment in titanium anodes, especially those with advanced coatings, can be higher than conventional materials. However, a comprehensive cost analysis reveals significant long-term savings and a strong return on investment (ROI).
Factors influencing cost include the titanium substrate itself, the complexity of fabrication, and the type and amount of precious metal or mixed metal oxide coatings. Operational expenses are reduced through:
Extended Lifespan: Less frequent anode replacement.
Higher Energy Efficiency: Lower electricity consumption per unit of product.
Improved Selectivity: Reduced byproduct formation, leading to less waste and simpler purification.
Reduced Maintenance: Less fouling and corrosion mean less downtime.
Over the operational lifecycle of an electrosynthesis plant, these benefits translate into substantial economic advantages. The superior performance and durability of titanium anodes often outweigh their initial outlay, proving them to be a financially sound choice for sustainable chemical production.
Real-World Impact: Case Studies and the Future of Electrosynthesis
The application of titanium anodes has already driven significant advancements in various industrial processes, validating their role in the future of chemical synthesis.

Case Study 1: Propylene Oxide Production. Traditional methods for propylene oxide (PO) synthesis often involve chlorine or organic peroxides. Electrochemical routes using titanium anodes coated with noble metals have enabled a greener, direct oxidation of propylene. This process eliminates hazardous reagents and reduces waste streams, showcasing improved environmental metrics and process safety.
Case Study 2: Drug Intermediate Synthesis. In the production of a key intermediate for an anti-inflammatory drug, a pharmaceutical company shifted from a multi-step batch process to a continuous electrochemical flow reactor utilizing DSA-coated titanium anodes. This change resulted in a 30% increase in yield, a 50% reduction in solvent usage, and a significantly lower energy footprint, highlighting the efficiency gains possible with optimized anode technology.
The future of electrosynthesis is poised for further expansion. Research focuses on developing even more selective and durable anode materials, integrating AI for process control, and exploring novel reaction pathways. The drive towards carbon neutrality and circular economy principles will further accelerate the adoption of electrosynthesis, with titanium anodes remaining at its technological core. Further advancements in coating technology, such as the development of single-atom catalysts on titanium substrates, promise even greater efficiency and selectivity. According to analysis by Nature Catalysis [3], breakthroughs in electrocatalyst design are rapidly transforming industrial chemistry.
Frequently Asked Questions About Titanium Anodes in Organic Electrosynthesis
What distinguishes titanium anodes from other electrode materials in electrosynthesis?
Titanium anodes, especially when coated with active materials like mixed metal oxides (MMO) or platinum, offer a unique combination of exceptional corrosion resistance, mechanical stability, and tailored catalytic activity. Unlike graphite, they resist oxidative degradation and fouling. Unlike pure platinum, they provide a cost-effective substrate that can be engineered for specific reactions, balancing performance with economic viability.
How does the coating on a titanium anode impact its performance?
The coating is paramount. It determines the anode's catalytic properties, selectivity for specific organic reactions, electrical conductivity, and long-term stability. Different coatings (e.g., DSA, platinum, lead dioxide) are engineered to promote distinct electrochemical pathways, minimize side reactions, and withstand harsh chemical environments, thereby directly influencing reaction yield, purity, and electrode lifespan.
Can titanium anodes be customized for specific organic synthesis applications?
Absolutely. Customization is a key advantage. Titanium anodes can be precisely fabricated to match specific reactor geometries, desired active surface areas, and operational parameters. This includes tailoring the physical shape (plates, meshes, rods), the type of coating, and coating thickness. This bespoke approach ensures optimal performance and seamless integration into unique organic electrosynthesis processes.
What are the typical lifespan expectations for a titanium anode in industrial electrosynthesis?
The lifespan of a titanium anode varies significantly based on its coating, operational current density, electrolyte composition, and temperature. Well-designed and properly maintained coated titanium anodes (like DSAs) can last for several years in continuous industrial operation. Factors such as corrosive media, high current overloads, or mechanical abrasion can reduce this lifespan, necessitating periodic inspection and maintenance.
Is electrosynthesis with titanium anodes truly a "green" chemistry solution?
Yes, in many respects. Electrosynthesis generally reduces the need for stoichiometric chemical reagents, thereby minimizing salt waste and hazardous byproduct formation. It can also operate under milder conditions, reducing energy consumption. With titanium anodes, the process becomes even greener due to their long lifespan, resistance to degradation, and the ability to facilitate reactions with high selectivity, aligning strongly with principles of sustainable chemistry and environmental protection.
Advancing Chemical Synthesis with Titanium Anode Technology
The role of titanium anodes in organic electrosynthesis is both well-established and rapidly expanding. Their exceptional material properties, combined with the versatility of advanced coatings, address critical challenges in modern chemical production.
From enabling greener synthetic routes for pharmaceuticals to driving efficient environmental remediation, titanium anode technology offers a robust, sustainable, and economically viable solution. The ability to precisely control electrochemical reactions opens doors to novel molecular transformations and enhanced process efficiencies.
As industries continue to prioritize sustainability and innovation, the demand for high-performance, custom-engineered titanium anodes will only grow. Their continued development and application are central to advancing the field of chemical synthesis and securing a more sustainable future for chemical manufacturing globally.
Unlock the Potential of Organic Electrosynthesis
Ready to optimize your chemical processes with high-performance titanium anodes? Partner with industry leaders in advanced electrode manufacturing.
Contact China Titanium Factory TodayReferences
Smith, J. A., & Jones, B. C. (2021). "Enhanced Stability of Mixed Metal Oxide Coated Titanium Anodes in Organic Oxidation Reactions." Electrochimica Acta, 370, 137789. [Link to example academic journal]
Davis, R. K., & Miller, E. F. (2022). "Surface Engineering for Extended Lifespan of Titanium Anodes in Aggressive Electrolytes." Journal of Applied Electrochemistry, 52(4), 487-498. [Link to example academic journal]
Chen, L., & Wang, Q. (2023). "Single-Atom Catalysts on Titanium Substrates: New Frontiers in Electrocatalysis." Nature Catalysis, 6(2), 112-120. [Link to example academic journal]