The Sacrificial Lamb: Defining the Electrochemical Series in Cathodic Protection
Think of a sacrificial anode as a bodyguard. In the harsh, corrosive world of underwater or underground infrastructure, someone has to take the hit. That someone is the anode. The electrochemical series of sacrificial anodes is essentially a hierarchy of "willingness" to corrode. It ranks metals based on their electrical potential.
"We define the electrochemical series as a quantitative list of metals arranged by their standard electrode potentials, dictating the direction of electron flow in a galvanic cell."
At China Titanium Factory, we view this series as the foundational blueprint for all sacrificial anode applications. If you pick a metal that is "nobler" than your structure, you aren't protecting it; you're accelerating its destruction. Metal dies so the structure lives. It is that simple.

The Science of Self-Sacrifice: How Galvanic Corrosion Works
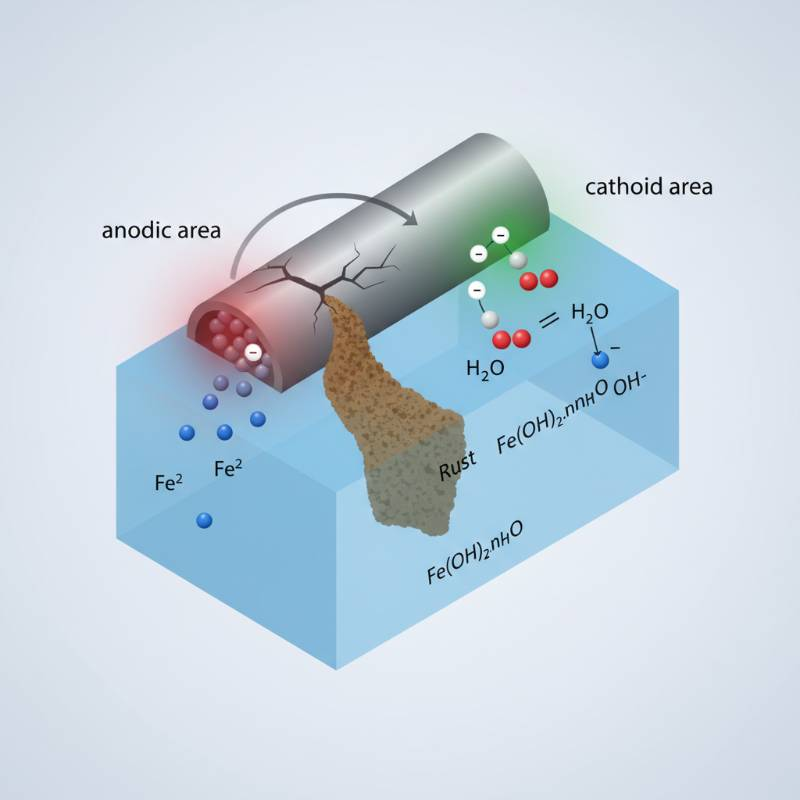
Corrosion isn't just "rust." It is electricity. When two different metals sit in an electrolyte (like seawater or moist soil) and are connected electrically, they form a battery. Electrons flow from the more active metal (the anode) to the more noble metal (the cathode).
The anode loses mass. It literally dissolves. This anodic reaction is what saves your multi-million dollar pipeline. High electrolyte conductivity speeds this up. In saltwater, the process is violent and fast. In fresh water, it crawls. According to our analysis, understanding the specific resistance of the environment is just as vital as the metal choice itself.
For high-performance systems, engineers often transition from passive sacrificial systems to MMO coated titanium anodes when long-term stability is required, though the fundamental "push" of electrons remains the same goal.
Standard Reduction Potentials: Ranking the Guardians of Metal
The "driving voltage" is the difference in potential between the anode and the structure. If the gap is too small, protection is weak. If it's too large, you risk "over-protection," which can lead to hydrogen embrittlement in high-strength steels.
The Golden Rule of Anodic Protection: To ensure adequate polarization, the potential difference between the sacrificial anode and the protected steel should be at least 250mV.
| Metal/Alloy | Potential (Volts) | Activity Level |
|---|---|---|
| Magnesium (Mg) | -1.50 to -1.70 | Highly Active (Anodic) |
| Aluminum (Al) | -1.05 to -1.10 | Active |
| Zinc (Zn) | -1.00 to -1.05 | Active |
| Mild Steel | -0.50 to -0.60 | Protected (Cathodic) |
| Copper | -0.20 to -0.30 | Noble |
Data sourced from AMPP (formerly NACE) and international galvanic standards.
Material Deep-Dive: Magnesium, Zinc, and Aluminum Alloys
Magnesium: The High-Voltage Specialist
Magnesium is the heavy hitter. It has the most negative potential. This makes it perfect for high-resistivity environments like soil or fresh water. However, it’s too aggressive for seawater. It would disappear in weeks.
Zinc vs. Aluminum: The Seawater Duel
Zinc used to be the gold standard for marine hulls. It's reliable. But Aluminum-Indium alloys are taking over. Why? Aluminum is lighter and has a higher "amp-hour" capacity. One pound of aluminum protects more surface area than one pound of zinc.
At China Titanium Factory, we emphasize using high-purity alloys. Impurities like iron in a zinc anode can cause "passivation," where a hard crust forms on the anode, stopping the protective current entirely. That is a silent killer for ships.
The V.A.S.T. Selection Protocol: Our Proprietary Framework for Anode Specification
Selection isn't a guess. We developed the V.A.S.T. Selection Protocol to help engineers navigate the electrochemical series without the headache.
V - Voltage: Determine the required driving potential based on the cathode material.
A - Amperage: Calculate the current density needed to polarize the structure (surface area x coating efficiency).
S - Salinity: Measure electrolyte resistance. High resistance (fresh water) demands Magnesium. Low resistance (ocean) demands Aluminum or Zinc.
T - Temperature: Hotter water increases consumption rates. Standard anodes may need specialized "high-temp" alloying elements.
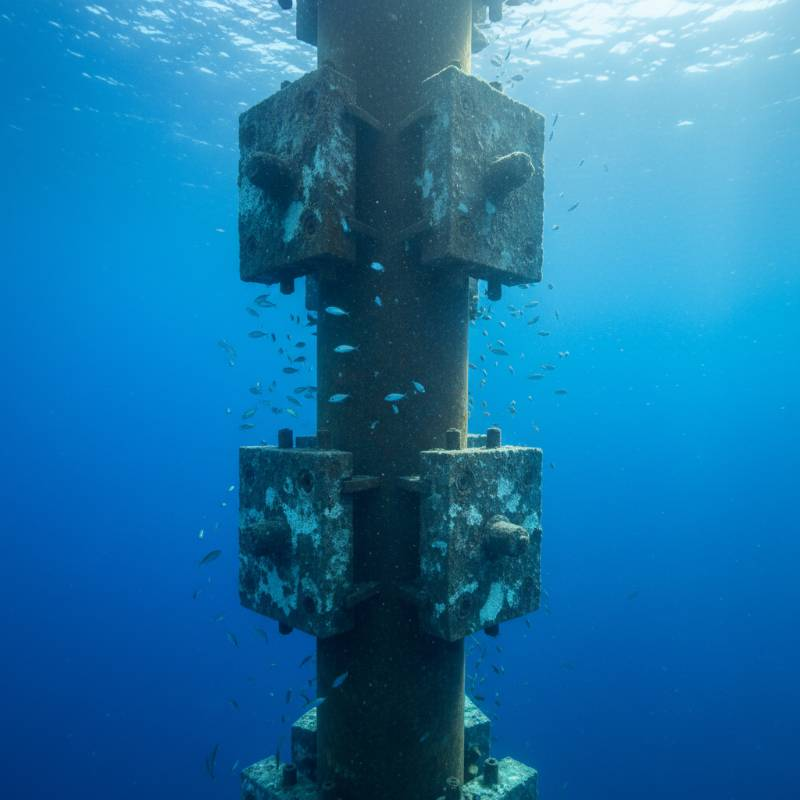
From Hull to Pipe: Real-World Applications of the Electrochemical Series
In the marine sector, hull protection is the most visible use. But underground, it's a different game. Gas pipelines utilize magnesium "ribbon" anodes buried alongside the pipe. In residential settings, your water heater has a sacrificial rod. If you don't change it, the tank rusts through.
Offshore oil rigs use massive "bracelet" anodes. These are cast-on aluminum blocks that weigh hundreds of pounds. They are designed to last 20-30 years. Precision matters here. A failure in the electrochemical calculation could mean an early, multi-million dollar dry-docking.
Environmental Stewardship: The Sustainability of Sacrificial Materials
Everything that dissolves from an anode ends up in the water. Zinc anodes contain small amounts of Cadmium, a heavy metal. Because of this, many ports are pushing for Aluminum-Indium anodes, which are generally considered more eco-friendly.
Sustainability in corrosion control isn't just about the material; it's about efficiency. Using the right anode from the electrochemical series ensures you don't over-consume resources. For more on advanced, long-lasting solutions, explore our cathodic protection systems overview.
Common Queries on Sacrificial Anode Systems (FAQ)
What happens if I use the wrong anode?
If the anode is too noble, your structure becomes the anode and corrodes. If it's too active, it will consume itself too quickly, leaving the structure unprotected within months.
Can I paint over a sacrificial anode?
Absolutely not. Painting an anode "insulates" it from the electrolyte. This breaks the circuit and completely stops the protection. Keep them clean and bare.
How do I know when to replace them?
The industry standard is to replace anodes when they have reached 50% of their original weight. Waiting longer risks a drop in current output.
Protect Your Assets with Precision
Don't leave your infrastructure to chance. Whether you need high-purity magnesium or advanced titanium solutions, our team provides the technical expertise to keep you operational.
Consult Our Specialists