Introduction: Understanding Titanium Anodizing and Its Environmental Context
Titanium, lauded for its exceptional strength-to-weight ratio, corrosion resistance, and biocompatibility, finds extensive application across aerospace, biomedical, automotive, and architectural sectors. Enhancing these intrinsic properties often involves surface treatments, with titanium anodizing standing out as a critical process.
Anodizing creates a stable, protective oxide layer on the titanium surface, improving wear resistance, hardness, and aesthetic appeal. However, the industrial processes involved are not without their environmental footprint. The chemicals used in titanium anodizing present a complex challenge for manufacturers committed to sustainability and regulatory compliance.
This guide dives deep into the environmental impact of these chemicals. We'll explore the problems they pose, outline actionable solutions, and provide the technical proof necessary for engineers and materials scientists to make informed decisions. Understanding these nuances is no longer just good practice; it’s a business imperative.
The Titanium Anodizing Process: A Technical Overview
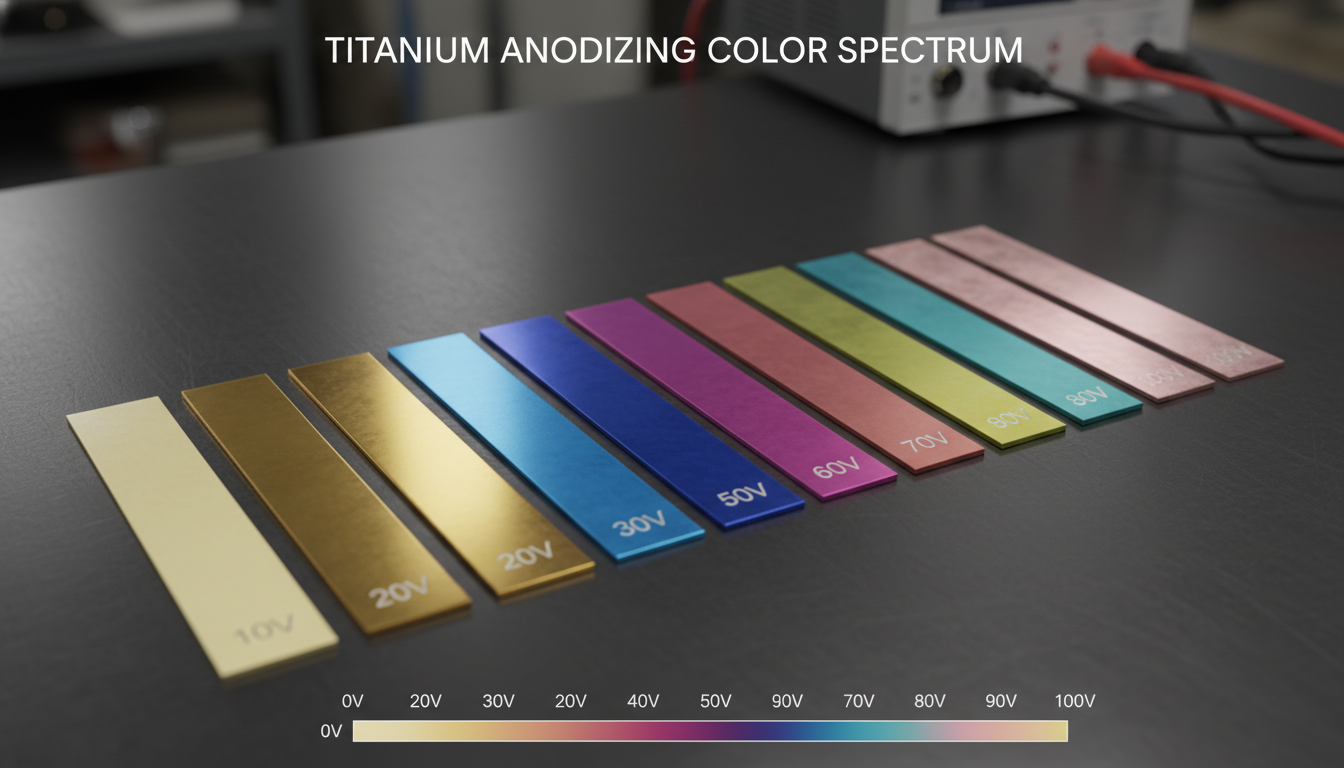
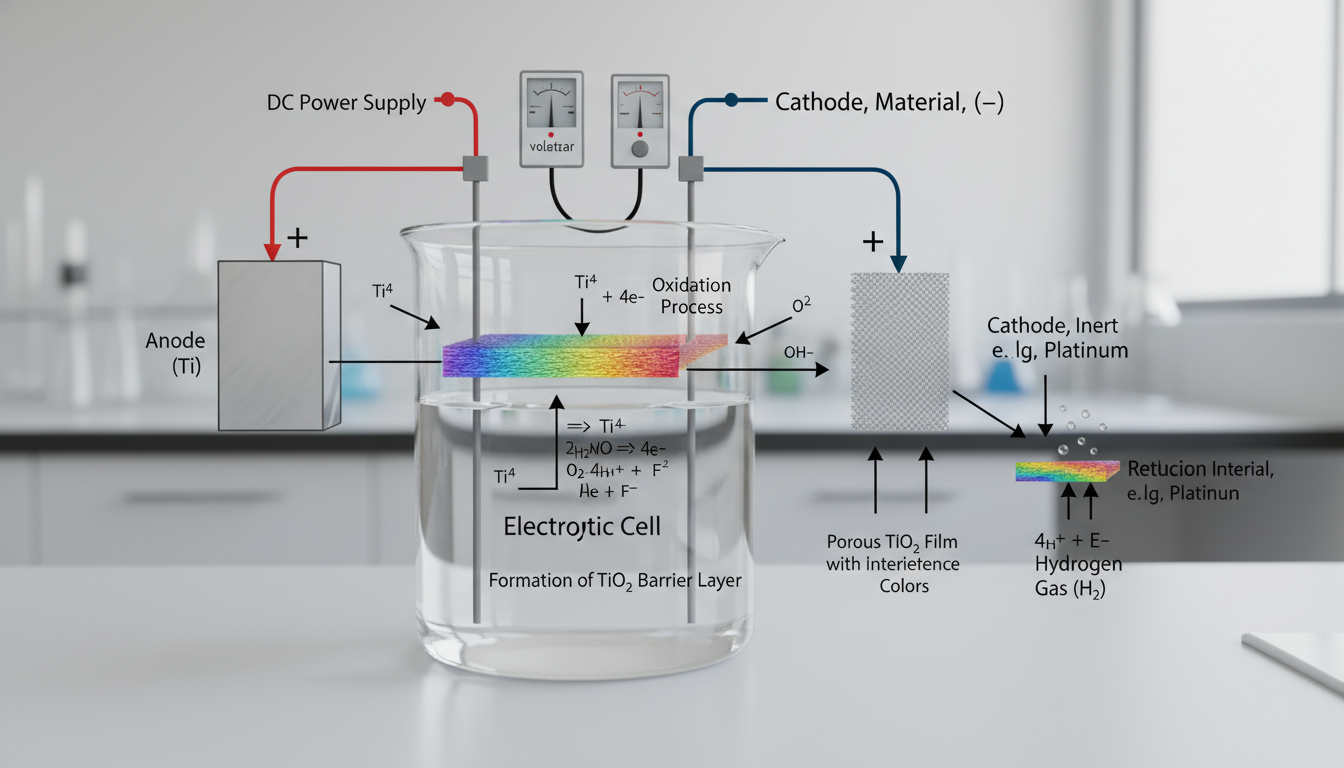
Titanium anodizing is an electrochemical passivation process. It converts the surface of titanium into a layer of titanium oxide. This oxide layer is significantly thicker and more durable than the naturally occurring passive film.
The workpiece, acting as the anode, is immersed in an electrolytic solution along with a cathode. A direct current passes through the bath. This current drives the controlled oxidation of the titanium surface.
The specific properties of the resulting oxide layer—thickness, porosity, and color—depend heavily on the electrolyte composition, voltage, current density, and processing time. Common electrolytes include various acidic solutions. These solutions facilitate the formation of the desired oxide film.
Classes of Chemicals Involved
The primary chemicals in an anodizing bath fall into a few categories:
Acids: These are the backbone of most anodizing electrolytes. Sulfuric acid, hydrofluoric acid, phosphoric acid, and chromic acid are frequently used, each yielding different surface characteristics.
Electrolytes: Beyond simple acids, specific salts or organic compounds might be added to fine-tune conductivity, film growth rate, and coloration. For instance, in Type II anodizing (often for color), phosphates or other proprietary blends are common.
Cleaning Agents: Before anodizing, titanium parts undergo rigorous cleaning to remove oils, grease, and surface contaminants. Caustic solutions, degreasers, and pickling agents often precede the anodizing step.
Each of these chemical classes brings its own set of handling, disposal, and environmental considerations to the table. For instance, the choice of custom titanium fabrication processes directly influences the subsequent anodizing requirements and associated chemical usage.
Hazardous Chemicals in Titanium Anodizing: A Deep Dive into Environmental Risks
The utility of certain chemicals in titanium anodizing often comes with significant environmental and health trade-offs. Identifying these hazardous components is the first step toward mitigation.
Hydrofluoric Acid (HF)
HF is highly effective for etching and surface preparation of titanium due to its ability to dissolve titanium oxide. However, its extreme corrosivity and toxicity are well-documented. Exposure can cause severe burns, systemic toxicity, and bone damage. In the environment, HF is corrosive to most materials and can acidify water bodies, harming aquatic life.
Hydrofluoric Acid (HF): A highly corrosive and toxic acid, unique in its ability to readily penetrate tissue and cause systemic toxicity by binding with calcium, leading to severe burns and potential cardiac arrest. It is also highly reactive with many materials, posing significant environmental release risks.
Proper containment and neutralization are paramount when handling HF, as even diluted solutions pose a serious threat.
Sulfuric Acid (H₂SO₄)
A common electrolyte in Type II and Type III anodizing, sulfuric acid is a strong mineral acid. It causes severe burns on contact and emits irritating fumes. Environmentally, sulfuric acid lowers pH levels in water and soil, impacting ecosystems. Acid spills can devastate local flora and fauna, altering nutrient cycles and heavy metal mobility. Its widespread use makes large-scale accidental releases a constant concern.
Chromates and Hexavalent Chromium
Historically, chromic acid (containing hexavalent chromium, Cr(VI)) was used for anodizing and sealing processes, offering excellent corrosion resistance. However, Cr(VI) is a known carcinogen, highly toxic, and persistent in the environment. It can contaminate groundwater, accumulate in the food chain, and cause severe health issues, including respiratory problems and organ damage. Due to these extreme risks, its use in many regions is heavily restricted or banned. Many manufacturers have shifted away from chromate-based titanium surface treatment processes.
For more detailed information on chromium toxicity, the Agency for Toxic Substances and Disease Registry (ATSDR) provides comprehensive resources on its public health statement. (Source 1)
Other Heavy Metals and Complexing Agents
While titanium itself is generally non-toxic, some anodizing solutions may contain other metallic salts or complexing agents. These can become problematic if discharged untreated. Heavy metals like nickel, cadmium, or lead, if present as impurities or additives, are persistent pollutants. Complexing agents can make it harder to remove these metals from wastewater through conventional precipitation methods.
Assessing the Environmental Footprint: Life Cycle Analysis and Impact Pathways
To truly grasp the environmental impact of titanium anodizing, a holistic approach is necessary. Life Cycle Assessment (LCA) provides a framework for quantifying impacts from cradle to grave. This includes raw material extraction, chemical manufacturing, the anodizing process itself, and waste disposal.
LCA Data and Key Impact Categories
LCA studies on metal finishing processes often highlight several critical impact categories:
Acidification: Primarily from sulfuric acid and other acidic emissions.
Eutrophication: Nutrient enrichment in water bodies, often from phosphates or nitrogen compounds in wastewater.
Human Toxicity: Direct exposure to hazardous chemicals, especially HF and Cr(VI).
Ecotoxicity: Harm to ecosystems, aquatic life, and soil organisms from chemical discharges.
Resource Depletion: Consumption of non-renewable resources, including chemicals and energy.
Water Depletion: Significant water usage in rinsing and bath replenishment.
The cumulative impact across these categories paints a sobering picture. It underscores the urgent need for process improvements.
Pollution Pathways and Their Effects
Chemicals from anodizing operations can enter the environment via several pathways:
Wastewater Discharge: Spent anodizing baths, rinse waters, and cleaning solutions contain dissolved metals, acids, and other chemicals. If inadequately treated, these effluents can contaminate rivers, lakes, and groundwater.
Air Emissions: Volatile organic compounds (VOCs) from cleaning agents, acid mists (e.g., sulfuric acid mist), and fumes from heated baths can be released into the atmosphere. These contribute to air pollution and can be transported over long distances.
Solid Waste/Sludge: Treatment of wastewater often generates hazardous sludge containing precipitated metals and other solidified contaminants. Improper disposal of this sludge can lead to soil and groundwater contamination.
Accidental Spills: Tank ruptures, handling errors, or leaks can result in direct release of concentrated chemicals into the environment, causing immediate and severe localized damage.

The environmental footprint is not just a regulatory hurdle. It reflects a tangible impact on ecosystems and human communities. Robust environmental management systems are essential for any responsible titanium manufacturer.
Waste Management and Disposal Strategies for Anodizing Byproducts
Dealing with hazardous waste from titanium anodizing isn't a walk in the park. It demands a systematic and technically sound approach. The goal is to minimize environmental harm and ensure regulatory compliance. This means getting down to brass tacks with treatment protocols.
Wastewater Treatment Protocols
Effective wastewater treatment is the cornerstone of responsible anodizing operations. Several techniques are employed, often in combination:
Neutralization: Acidic wastewater is typically neutralized to a pH range of 6-9 using alkaline agents like caustic soda (NaOH) or lime (Ca(OH)₂). This reduces corrosivity and precipitates many dissolved metals.
Precipitation: Following neutralization, heavy metals often precipitate as hydroxides. Coagulants and flocculants are then added to aggregate these fine particles into larger, settleable flocs.
Filtration: Various filtration methods, including sand filters, membrane filtration (microfiltration, ultrafiltration), or activated carbon filtration, remove suspended solids, precipitated metals, and some dissolved organic compounds.
Ion Exchange: This advanced technique uses resin beds to selectively remove specific ions, such as heavy metals or fluorides, from the wastewater stream, achieving very low discharge limits.
Sludge Handling and Disposal
The byproduct of most wastewater treatment is hazardous sludge. This solid waste contains concentrated heavy metals and other contaminants. Proper handling is critical:
Dewatering: Sludge is typically dewatered using filter presses or centrifuges to reduce its volume, making it easier and less costly to transport and dispose of.
Stabilization/Solidification: In some cases, sludge undergoes stabilization or solidification processes. This involves mixing it with binding agents (e.g., cement, lime) to reduce leachate potential and immobilize contaminants.
Hazardous Waste Landfills: Dewatered and stabilized sludge must be transported to permitted hazardous waste landfills for secure disposal. This ensures contaminants do not leach into surrounding soil or groundwater.
Adhering to these protocols is not just regulatory compliance; it's fundamental to environmental stewardship for any operation dealing with titanium products.
Exploring Safer Alternatives and Eco-Friendly Anodizing Processes
The push for sustainability has spurred innovation in anodizing chemistry. Engineers are actively seeking to replace the most problematic chemicals with safer, equally effective alternatives. This isn't just about regulatory avoidance; it's about getting ahead of the curve.
Replacing Hazardous Acids
Sulfuric Acid Alternatives: While sulfuric acid remains prevalent, research into organic acids (e.g., oxalic acid, tartaric acid, citric acid) and mixtures thereof shows promise. These can achieve similar oxide layer properties with reduced environmental impact, often generating less hazardous wastewater.
Hydrofluoric Acid (HF) Substitutes: Replacing HF in titanium etching and cleaning is a tougher nut to crack due to its unique reactivity. However, some processes are exploring combinations of less hazardous inorganic acids with fluoroboric acid or ammonium bifluoride at lower concentrations. Mechanical surface preparation methods are also being considered where applicable to reduce reliance on chemical etching.
Chromate-Free Anodizing and Sealing
The industry has largely moved away from hexavalent chromium due to its toxicity. Significant progress has been made in developing chromate-free alternatives:
Tartaric-Sulfuric Acid Anodizing (TSA): This process is a direct replacement for chromic acid anodizing in some applications, producing comparable corrosion resistance.
Boric-Sulfuric Acid Anodizing (BSA): Another chromate-free option, BSA offers good fatigue resistance, particularly important for aerospace components.
Non-Chromate Sealing: For enhancing corrosion resistance post-anodizing, alternatives like nickel acetate, cobalt acetate, or rare earth element-based seals are gaining traction. These offer robust performance without the Cr(VI) burden.
Plasma Electrolytic Oxidation (PEO)
PEO, also known as micro-arc oxidation (MAO), is an advanced electrochemical surface treatment that forms ceramic-like oxide layers on titanium. The process typically uses alkaline silicate or phosphate electrolytes, which are significantly less hazardous than traditional strong acid baths. PEO coatings offer superior hardness, wear resistance, and corrosion protection. This represents a significant leap forward in eco-friendly titanium manufacturing.
Plasma Electrolytic Oxidation (PEO): An electrochemical surface treatment process that forms a hard, ceramic-like oxide layer on light metals (including titanium) in an environmentally benign aqueous electrolyte, often involving localized plasma discharges to achieve excellent tribological and corrosion properties.
These eco-friendly anodizing solutions are not just theoretical; they are being implemented in industrial settings, proving that high performance and environmental responsibility can go hand-in-hand. The European Chemicals Agency (ECHA) provides extensive guidance on substituting hazardous chemicals, offering a strong external resource for businesses looking to transition. (Source 2)
Implementing Best Practices: Waste Minimization and Closed-Loop Systems
Simply treating waste after it's generated is only half the battle. The smarter play is to minimize waste at the source. This is where process optimization and closed-loop systems really shine, turning a cost center into an efficiency gain. It's about working smarter, not just harder.
Process Optimization for Waste Reduction
Bath Life Extension: Extending the operational life of anodizing baths reduces the frequency of dumping and replenishment. This can be achieved through continuous filtration to remove suspended solids, careful monitoring of chemical concentrations, and replenishment of depleted components rather than full bath changes.
Drag-Out Reduction: Minimizing the amount of solution carried out of the anodizing tank on the workpiece (drag-out) is crucial. Techniques include proper rack design, longer drain times, and the use of air knives to physically remove excess solution before rinsing.
Rinse Water Management: Implementing counter-flow rinsing or cascade rinsing systems significantly reduces fresh water consumption and the volume of wastewater requiring treatment. Each subsequent rinse tank uses water from the next cleaner stage, progressively reducing contaminant concentration.
Optimized Pre-Treatment: Efficient cleaning and etching prior to anodizing reduce contaminant loading in the main bath and subsequent rinse waters.
Closed-Loop Systems and Resource Recovery
The ultimate goal for sustainable anodizing is to achieve near-zero discharge through closed-loop systems. This involves recycling and reusing chemicals and water within the process:
Chemical Recycling: Technologies like membrane filtration (e.g., nanofiltration, reverse osmosis) or evaporation can recover valuable chemicals from spent baths or rinse waters. Acids can be concentrated and reused, reducing the need for virgin chemical purchases and minimizing hazardous waste volume.
Water Reuse: Treated wastewater, particularly from rinse stages, can be purified to a level suitable for reuse in other non-critical process steps or back into the rinse lines. This dramatically cuts down on fresh water intake and wastewater discharge volumes.
Electrodialysis: This electrochemical process can separate ionic components from solutions, allowing for the recovery of acids or metal salts and the purification of water for reuse.

Implementing these best practices requires an upfront investment, but the long-term savings in chemical costs, water usage, and waste disposal fees, combined with enhanced environmental standing, often provide a compelling return on investment. Businesses that embrace these principles often find themselves at the forefront of sustainable titanium fabrication.
Navigating the Regulatory Landscape: Compliance and Standards
Operating a titanium anodizing facility means playing by a stringent set of rules. Environmental regulations are complex, ever-evolving, and carry significant penalties for non-compliance. Keeping up with these mandates is a full-time job for many operations.
Key Environmental Regulations in the US
Clean Water Act (CWA): Governs wastewater discharges into navigable waters. Anodizing facilities typically require National Pollutant Discharge Elimination System (NPDES) permits, which set limits on pH, heavy metals, and other pollutants. Pretreatment standards for facilities discharging to Publicly Owned Treatment Works (POTWs) are also critical.
Resource Conservation and Recovery Act (RCRA): Regulates the generation, transportation, treatment, storage, and disposal of hazardous waste. Spent anodizing baths and treatment sludges often fall under RCRA hazardous waste classifications, necessitating strict cradle-to-grave management.
Clean Air Act (CAA): Addresses air emissions, including acid mists and volatile organic compounds. Facilities may need permits and implement controls to limit airborne pollutants.
Emergency Planning and Community Right-to-Know Act (EPCRA): Requires facilities to report on the storage, use, and releases of hazardous chemicals, providing information to local emergency responders and the public.
Global Standards and Industry-Specific Guidelines
Beyond national laws, international standards and industry-specific guidelines play a significant role:
REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals): A European Union regulation impacting manufacturers globally who export to the EU, placing strict controls on hazardous substances like hexavalent chromium.
ISO 14001: An international standard for environmental management systems (EMS). Certification demonstrates a company's commitment to minimizing its environmental footprint and continuously improving its environmental performance.
Industry Best Practices: Associations like the National Association for Surface Finishing (NASF) provide guidance and best practices for environmental compliance and sustainable operations in the metal finishing sector.
Staying compliant means more than avoiding fines; it builds trust with clients, regulators, and the community. Companies like China Titanium Factory are committed to adhering to these rigorous standards in their titanium operations.
The Economic Case for Sustainability: Cost-Benefit Analysis of Green Practices
Some view sustainable practices as an added cost, a necessary evil. But that's a shortsighted perspective. The reality is that green manufacturing in titanium anodizing often pays dividends. It’s not just about doing the right thing; it’s about making smart business decisions. The bottom line can actually improve.
Reduced Operational Costs
Lower Chemical Consumption: Implementing bath life extension and chemical recycling directly translates to less frequent purchases of expensive virgin chemicals.
Decreased Water Bills: Closed-loop water systems and efficient rinsing techniques drastically cut down on fresh water intake and wastewater discharge volumes, leading to significant savings on utility bills and discharge fees.
Reduced Waste Disposal Expenses: Minimizing hazardous waste generation means lower costs for specialized transportation, treatment, and landfilling. This is especially true for highly regulated wastes like Cr(VI) sludge.
Energy Efficiency: Modern, optimized processes often consume less energy for heating, cooling, and pumping, further reducing operational overhead.
Enhanced Brand Reputation and Market Access
In today's market, customers, investors, and supply chains increasingly demand environmental responsibility. A strong sustainability profile provides several benefits:
Competitive Advantage: Companies with certified green practices often win bids over competitors, especially in industries like aerospace and biomedical where environmental impact is closely scrutinized.
Customer Loyalty: Environmentally conscious consumers and businesses are more likely to choose suppliers demonstrating a commitment to sustainability.
Reduced Regulatory Risk: Proactive sustainability efforts often place a company ahead of evolving regulations, reducing the risk of fines, legal battles, and costly retrofits.
Access to Green Financing: Some financial institutions offer favorable terms for businesses investing in eco-friendly technologies.
The return on investment (ROI) for sustainability initiatives can be compelling. While initial capital expenditure for new equipment might seem steep, the long-term economic benefits, coupled with improved brand equity, make a strong case for integrating green practices into the core business strategy.
For further insights into the economic benefits of sustainable manufacturing, the Environmental Protection Agency (EPA) offers numerous resources and case studies. (Source 3)
Emerging Technologies and Future Outlook in Sustainable Anodizing
The field of sustainable anodizing is far from stagnant. Researchers and engineers are pushing the envelope, developing cutting-edge solutions that promise to further minimize environmental impact while maintaining or even enhancing performance. Tomorrow's anodizing processes will be greener, more efficient, and smarter.
Advanced Electrolytes and Nanotechnology
Ionic Liquids: These molten salts, liquid at room temperature, offer unique properties as electrolytes. They are non-volatile, reducing air emissions, and can often be recycled. Research is ongoing to harness their potential for forming highly tailored oxide layers on titanium with minimal waste.
Deep Eutectic Solvents (DES): Similar to ionic liquids, DES are considered greener alternatives due to their low toxicity and biodegradability. They offer tunable properties for specific anodizing applications.
Nanoparticle-Enhanced Electrolytes: Incorporating specific nanoparticles into anodizing baths can modify the growth and structure of the oxide layer, potentially leading to superior properties with less aggressive chemistry.
Smart Process Control and AI
The integration of advanced sensors, real-time data analytics, and artificial intelligence (AI) is set to revolutionize anodizing:
Predictive Maintenance: AI algorithms can analyze bath chemistry data to predict when replenishment or maintenance is needed, preventing premature bath dumps and optimizing chemical usage.
Automated Optimization: Machine learning can fine-tune process parameters (voltage, current, time, temperature) in real-time to achieve desired film properties with minimal resource consumption and waste generation.
Reduced Human Error: Automation and smart controls reduce the likelihood of costly and environmentally damaging human errors.

These emerging technologies represent the next frontier in sustainable titanium anodizing. They promise not only to further mitigate environmental impact but also to enhance process control, product quality, and overall operational efficiency. The future of titanium surface treatment is undeniably green and smart.
Frequently Asked Questions (FAQs) about Titanium Anodizing and Environmental Impact
What are the most environmentally problematic chemicals in titanium anodizing?
The most problematic chemicals are typically hydrofluoric acid (HF), sulfuric acid, and historically, hexavalent chromium compounds. HF and sulfuric acid are highly corrosive and toxic, posing risks to both human health and ecosystems if not managed correctly. Hexavalent chromium is a known carcinogen and is heavily regulated due to its extreme toxicity and persistence.
Can titanium anodizing be done without hazardous chemicals?
While completely eliminating all hazardous chemicals is challenging, significant progress has been made. Alternatives like Plasma Electrolytic Oxidation (PEO) use less hazardous alkaline silicate or phosphate electrolytes. Many facilities now use chromate-free anodizing and sealing solutions, such as tartaric-sulfuric acid anodizing, which are much safer. The industry is constantly researching and adopting greener chemistry.
What are closed-loop systems, and how do they benefit environmental sustainability?
Closed-loop systems aim to recycle and reuse process chemicals and water within the manufacturing operation, minimizing external discharge. Benefits include significantly reduced fresh water consumption, lower chemical purchasing costs, decreased hazardous waste generation, and reduced environmental impact. Technologies like membrane filtration, ion exchange, and evaporation are key components of these systems.
How do regulations impact titanium anodizing operations?
Regulations like the Clean Water Act, RCRA, and Clean Air Act dictate stringent limits on wastewater discharge, hazardous waste disposal, and air emissions. Compliance requires permits, regular monitoring, and often significant investment in wastewater treatment and waste management infrastructure. Non-compliance can lead to substantial fines, operational shutdowns, and reputational damage.
Is investing in eco-friendly anodizing practices economically viable?
Absolutely. While there may be upfront capital costs, the long-term economic benefits are substantial. These include reduced operational costs from lower chemical and water consumption, decreased waste disposal expenses, and improved energy efficiency. Beyond direct savings, eco-friendly practices enhance brand reputation, provide a competitive advantage, reduce regulatory risks, and can even open doors to new markets and financing opportunities.