Introduction to Metal Surface Treatments: Heat Coloring and Electrolytic Anodizing
Engineers and material scientists constantly face the challenge of selecting the right surface treatment for metal components. The stakes are high. An incorrect choice can compromise performance, aesthetics, and component longevity, leading to costly failures. Two prevalent methods for altering metal surfaces are heat coloring and electrolytic anodizing. While both can impart color and some level of protection, their underlying mechanisms, resulting properties, and ideal applications differ significantly.
Understanding these distinctions is crucial for optimal material selection and process specification. This guide cuts through the noise, offering a deep technical dive into both processes. We'll compare them directly, helping you navigate the complexities and pinpoint the best solution for your project. For advanced metal fabrication needs, especially involving precision titanium, a thorough understanding of these treatments is indispensable.
Understanding the Heat Coloring Process
Heat coloring, also known as thermal oxidation or bluing, is a time-honored technique. It involves controlled heating of a metal surface in a specific atmosphere to induce a thin oxide layer. This process changes the metal's appearance and can offer minor protective qualities.
What is Heat Coloring?
Heat coloring is a surface treatment process where a metal is intentionally heated to precise temperatures, often in the presence of air or a controlled atmosphere, to form a thin, adherent oxide layer that imparts a distinct color to the surface.
The technique has roots in historical metalwork, notably for firearms. Modern applications leverage its aesthetic appeal and the subtle changes it brings to surface properties. Think of the deep blues on a finely crafted watch spring or the rich browns on certain steel components.
The Science Behind Heat Coloring: How it Works
At its core, heat coloring is a process of controlled corrosion. As the metal heats, oxygen from the surrounding atmosphere reacts with the metal surface, forming an oxide layer. The thickness of this oxide layer dictates the observed color, not the pigment. This phenomenon is called thin-film interference.
Light interacts with the top surface of the oxide layer and the interface between the oxide and the base metal. When these reflected light waves interfere with each other, certain wavelengths are enhanced or canceled, producing specific colors. Temperature, heating duration, and atmospheric composition are critical variables. Even a slight deviation can dramatically alter the final hue. For instance, steel exposed to progressively higher temperatures cycles through straw, brown, purple, blue, and gray.
For more detailed insights into thin-film optics, a resource like SPIE Digital Library offers extensive research on optical properties of thin films.
Metals Suited for Heat Coloring
While various metals can be heat colored, some respond more predictably and effectively:
Steel: Classic bluing for firearms and tools is a prime example. The iron oxides (magnetite) create durable, aesthetically pleasing finishes.
Stainless Steel: Can be heat colored, though the results may vary more depending on alloy composition. Achieving uniform color can be tricky.
Titanium: Titanium's natural affinity for forming stable oxide layers makes it an excellent candidate for heat coloring. The colors range from gold to blue to purple, depending on temperature. This method is simpler than anodizing for achieving certain colors but offers less control over oxide thickness. Titanium forging components can often benefit from this treatment for specific cosmetic or identification purposes.
Advantages and Limitations of Heat Coloring
Heat coloring offers a unique set of benefits and drawbacks:
Advantages:
Aesthetic Versatility: Produces a spectrum of colors without dyes, often with a unique, organic feel.
Simplicity: Can be relatively straightforward compared to electrochemical processes, requiring less specialized equipment for basic applications.
Cost-Effective: For small batches or specific metals, it can be less expensive than other finishing methods.
Dimensional Stability: The oxide layer is extremely thin, causing minimal to no dimensional change to the part.
Limitations:
Limited Corrosion Resistance: The oxide layer is typically very thin, offering only minimal protection against corrosion. It's not a substitute for robust corrosion coatings.
Poor Wear Resistance: The thin oxide layer provides little to no enhancement in wear resistance.
Process Control: Achieving consistent, repeatable colors across batches can be challenging, particularly without highly controlled environments.
Material Dependence: Not all metals respond well, and results vary significantly with alloy composition.
Heat Distortion Risk: Heating can induce stress or distortion in thin or complex parts.
Key Applications of Heat Coloring
Despite its limitations, heat coloring finds its niche in several areas:
Firearm Bluing: A classic application for aesthetic appeal and very mild rust prevention.
Artistic Metalwork: Sculptors and jewelers use it for unique finishes on steel, copper, and titanium.
Decorative Components: Small, interior parts in consumer electronics or luxury goods where visual appeal is paramount but high durability isn't critical.
Medical Instruments (limited): For identification or specific aesthetic requirements on certain titanium components, where sterilization methods are compatible.

Exploring the Electrolytic Anodizing Process
Electrolytic anodizing is a robust electrochemical conversion process. Unlike heat coloring, it actively builds a thicker, more protective oxide layer. This layer is integral to the substrate metal, not merely a coating, offering superior functional properties.
What is Electrolytic Anodizing?
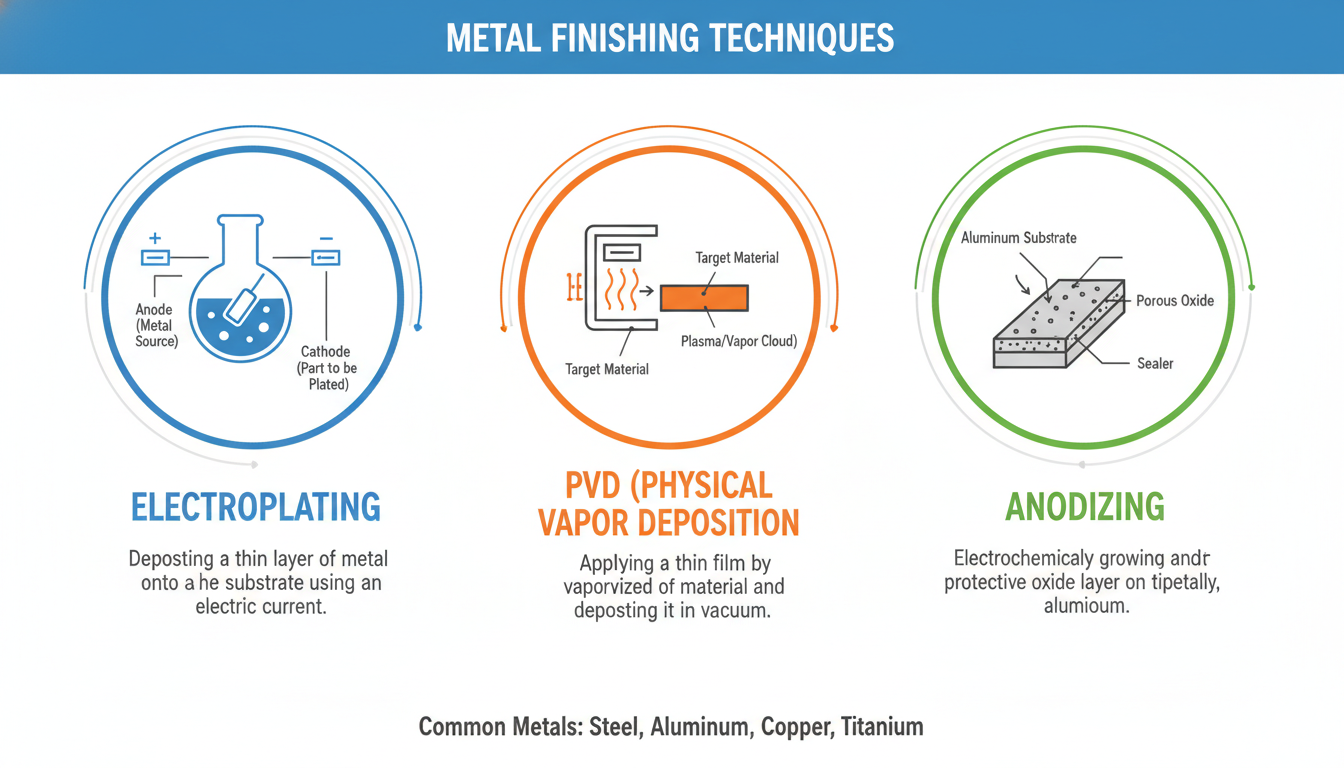
Electrolytic anodizing is an electrochemical passivation process used to increase the thickness of the natural oxide layer on the surface of metal parts. It enhances corrosion resistance, wear resistance, and allows for decorative coloring.
The term "anodizing" comes from the fact that the part being treated forms the anode electrode of an electrical circuit. This controlled oxidation is a cornerstone of modern metal finishing services for critical applications.
The Anodizing Process Explained
The anodizing process typically follows these steps:
Pre-treatment: Parts are meticulously cleaned to remove oils, dirt, and existing oxide layers. This often involves alkaline cleaning, rinsing, and acid etching.
Anodizing Bath: The cleaned metal part is immersed in an electrolytic solution (e.g., sulfuric acid, chromic acid, phosphoric acid).
Electrochemical Oxidation: A direct electric current is passed through the solution. The metal part acts as the anode, attracting negatively charged oxygen ions from the electrolyte. These ions react with the metal surface to form a dense, porous oxide layer.
Rinsing: Parts are thoroughly rinsed to remove residual electrolyte.
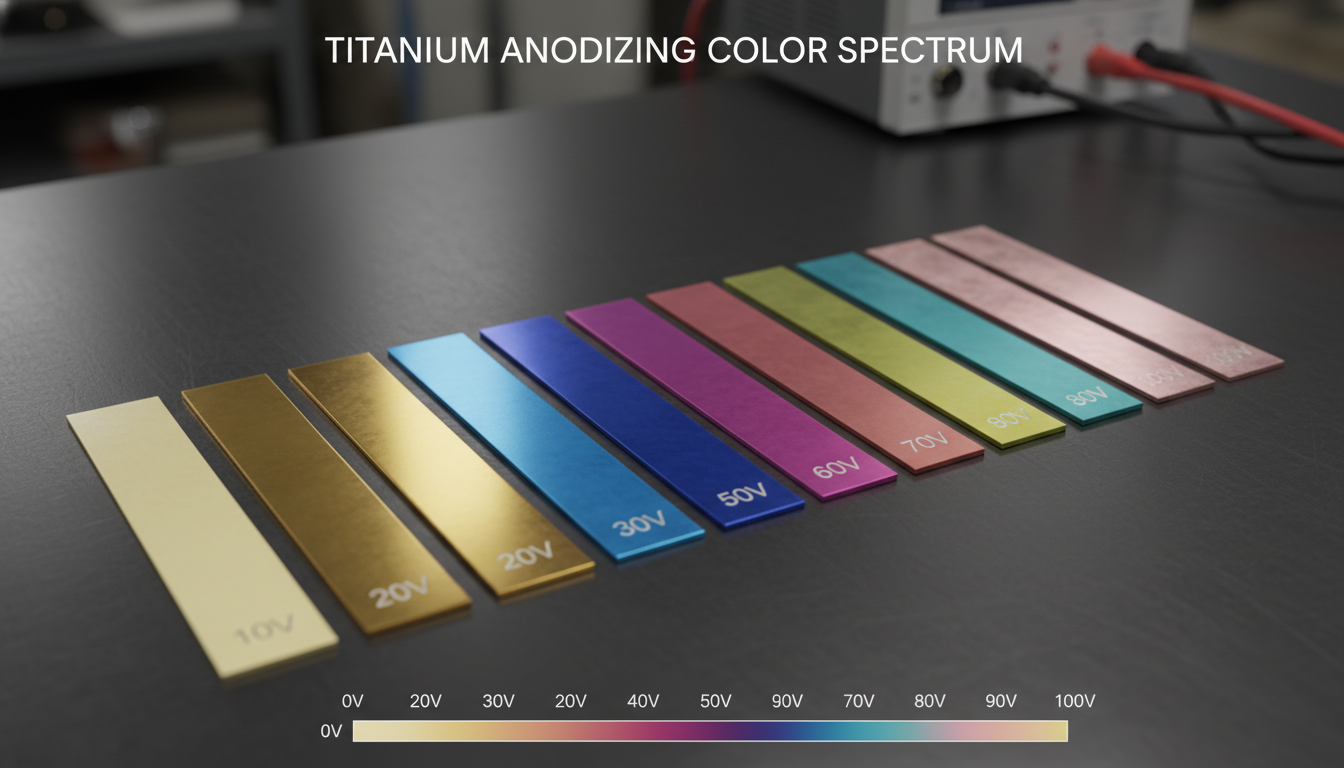
Coloring (Optional): For decorative finishes, the porous oxide layer can be dyed with organic or inorganic pigments. Alternatively, interference colors can be achieved by varying oxide thickness, particularly for titanium.
Sealing: The pores in the oxide layer are sealed, either by hydration (hot water or steam) or by chemical sealing agents. Sealing significantly improves corrosion resistance and prevents dye bleed-out.
The voltage, current density, electrolyte type, and temperature are precisely controlled to achieve specific oxide layer characteristics.
Metals Suited for Anodizing (Aluminum, Titanium, and More)
While commonly associated with aluminum, anodizing is effective for several other non-ferrous metals:
Aluminum: The most common substrate. Anodizing aluminum creates an extremely hard, corrosion-resistant surface ideal for countless industrial and consumer products.
Titanium: Highly amenable to anodizing, producing vibrant interference colors (without dyes) and enhancing biocompatibility and corrosion resistance. This makes titanium anodizing invaluable for medical implants, aerospace components, and aesthetic applications.
Magnesium: Can be anodized to improve corrosion and wear resistance, though it's a more specialized process.
Niobium: Similar to titanium, niobium anodizes to produce brilliant interference colors, often used in jewelry and art.
Types of Anodizing: From Decorative to Hardcoat (Type I, II, III)
The specific properties imparted by anodizing depend on the process type:
Type I (Chromic Acid Anodizing): Produces a thin, dense, opaque oxide layer. Offers good corrosion resistance and is preferred for fatigue-sensitive parts and complex geometries due to minimal dimensional change. It's less porous and generally not dyed.
Type II (Sulfuric Acid Anodizing - "Regular Anodize"): The most common type. Creates a thicker, more porous oxide layer suitable for dyeing a wide range of colors. Provides excellent corrosion resistance and improved wear properties. This is often used for general decorative and protective purposes.
Type III (Hardcoat Anodizing - "Hard Anodize"): Achieved by using lower temperatures and higher current densities in sulfuric acid. Produces the thickest, hardest, and densest oxide layer. Offers exceptional wear resistance, abrasion resistance, and dielectric strength. It's often dark gray or bronze, though it can be dyed. Ideal for components subjected to extreme wear and harsh environments.
ASTM B580 is a key standard specifying the engineering requirements for anodic oxide coatings on aluminum alloys. Referencing such standards is crucial for material scientists. You can find more details on such standards at ASTM International.
Advantages and Limitations of Electrolytic Anodizing
Anodizing brings substantial benefits, but also specific considerations:
Advantages:
Superior Corrosion Resistance: The thick, integral oxide layer provides excellent barrier protection against environmental degradation.
Enhanced Wear Resistance: Especially with Type III hardcoat, anodizing significantly increases surface hardness and abrasion resistance.
Aesthetic Versatility: Wide range of colors possible (especially with Type II) and interference colors for titanium.
Electrical Insulation: The oxide layer is non-conductive, offering good dielectric properties.
Improved Adhesion: Provides an excellent base for paints and primers.
Biocompatibility (Titanium): Anodized titanium is highly biocompatible, making it suitable for medical and dental implants.
Integral Finish: The oxide layer grows out of the base metal, meaning it won't chip or peel like plated coatings.
Limitations:
Material Specificity: Primarily effective on aluminum, titanium, magnesium, and niobium. Not suitable for ferrous metals.
Dimensional Change: The oxide layer adds thickness, which must be accounted for in tight tolerance precision machining.
Cost: Can be more expensive than heat coloring due to equipment, chemicals, and precise process control requirements.
Environmental Concerns: Some anodizing baths involve hazardous chemicals requiring careful waste management.
Fatigue: Thick anodic coatings, especially hardcoat, can reduce the fatigue strength of aluminum alloys.
Key Applications of Electrolytic Anodizing
Anodizing is a workhorse across numerous high-performance industries:
Aerospace: Critical components in aircraft and spacecraft benefit from enhanced corrosion and wear resistance.
Automotive: Decorative trim, engine components, and brake calipers.
Medical Devices: Surgical instruments, implants, and prosthetics often utilize titanium anodizing service for biocompatibility and specific coloring for identification.
Consumer Electronics: Smartphone casings, laptop bodies, and camera parts for aesthetics and durability.
Architectural: Building facades, window frames, and decorative panels.
Sporting Goods: Bicycle frames, camping gear, and fishing reels.

Heat Coloring vs. Electrolytic Anodizing: A Direct Technical Comparison
Now, let's get down to brass tacks. While both techniques modify a metal's surface, their fundamental differences dictate their suitability for various applications. Choosing between them means evaluating core properties.
Fundamental Differences in Process and Mechanism
The crux of the distinction lies in how the oxide layer is formed:
Heat Coloring: A thermal process. Oxidation occurs as oxygen from the air reacts directly with the hot metal surface. It's a spontaneous chemical reaction accelerated by heat. The resulting oxide layer is typically very thin and non-porous.
Electrolytic Anodizing: An electrochemical process. The metal is deliberately oxidized in an electrolyte bath using an external electrical current. This controlled environment allows for the growth of a thicker, often porous, and highly uniform oxide layer with tailored properties.
Comparative Analysis: Durability, Aesthetics, and Performance
Here’s a side-by-side look at key performance metrics:
Cost and Equipment Considerations
When budgeting for surface finishing, both upfront and operational costs matter:
Heat Coloring: Requires controlled ovens or torches. Initial setup can be relatively low-cost for basic operations. Operational costs are primarily energy for heating and labor. Scalability for high-volume, consistent output can be challenging without advanced automation.
Electrolytic Anodizing: Demands more significant investment in tanks, rectifiers, chillers, filtration systems, and chemical management. Operational costs include electricity, chemicals, waste treatment, and skilled labor. However, for high-volume, precision applications, the per-unit cost can be competitive due to efficiency and superior properties.
Environmental Impact and Safety Protocols
Both processes have environmental and safety footprints:
Heat Coloring: Primarily involves energy consumption and potential air emissions from heating (e.g., if oils or contaminants are present). Safety concerns revolve around high temperatures and proper ventilation.
Electrolytic Anodizing: Involves various acids and chemicals. This necessitates strict waste treatment protocols, ventilation, and personal protective equipment (PPE). The environmental impact comes from chemical disposal and energy usage.
Compliance with environmental regulations, such as those from the U.S. Environmental Protection Agency (EPA), is non-negotiable for anodizing facilities.
Choosing the Optimal Surface Treatment for Your Application
Making the right call between heat coloring and electrolytic anodizing isn't just about preference. It's about aligning the process with your component's functional requirements, aesthetic goals, and cost parameters. Think of it as hitting the bullseye on your project specifications.
Factors Influencing Your Decision
Consider these critical factors:
Substrate Material: Is it aluminum, titanium, steel, or another alloy? This is often the first filter.
Required Finish Properties: Do you need high corrosion resistance, superior wear resistance, electrical insulation, or primarily aesthetics?
Aesthetic Goals: Do you need a wide range of consistent colors, or a more subtle, natural oxide appearance?
Dimensional Tolerances: Can your part accommodate a micron-level thickness increase from anodizing?
Budget and Volume: What's your per-part cost target, and what's the production scale?
Environmental and Safety Concerns: Are there specific constraints on chemical usage or waste generation?
Post-Processing: Will the part be painted, bonded, or further processed?
Specific Use Cases and Recommendations
For high-performance, critical components (e.g., aerospace, medical implants, high-wear industrial parts): Electrolytic anodizing is almost always the go-to. Its controlled, durable oxide layer offers predictable corrosion and wear resistance. For custom titanium parts, anodizing ensures both functional integrity and aesthetic appeal.
For decorative steel or artistic pieces where extreme durability isn't the primary concern: Heat coloring can provide a unique, cost-effective finish.
For internal components requiring minor corrosion protection or identification: Heat coloring might suffice if the operating environment is mild.
When dimensional stability is paramount and color is a secondary, subtle requirement on titanium: Both can be considered, but anodizing offers more precise control over the interference color spectrum.
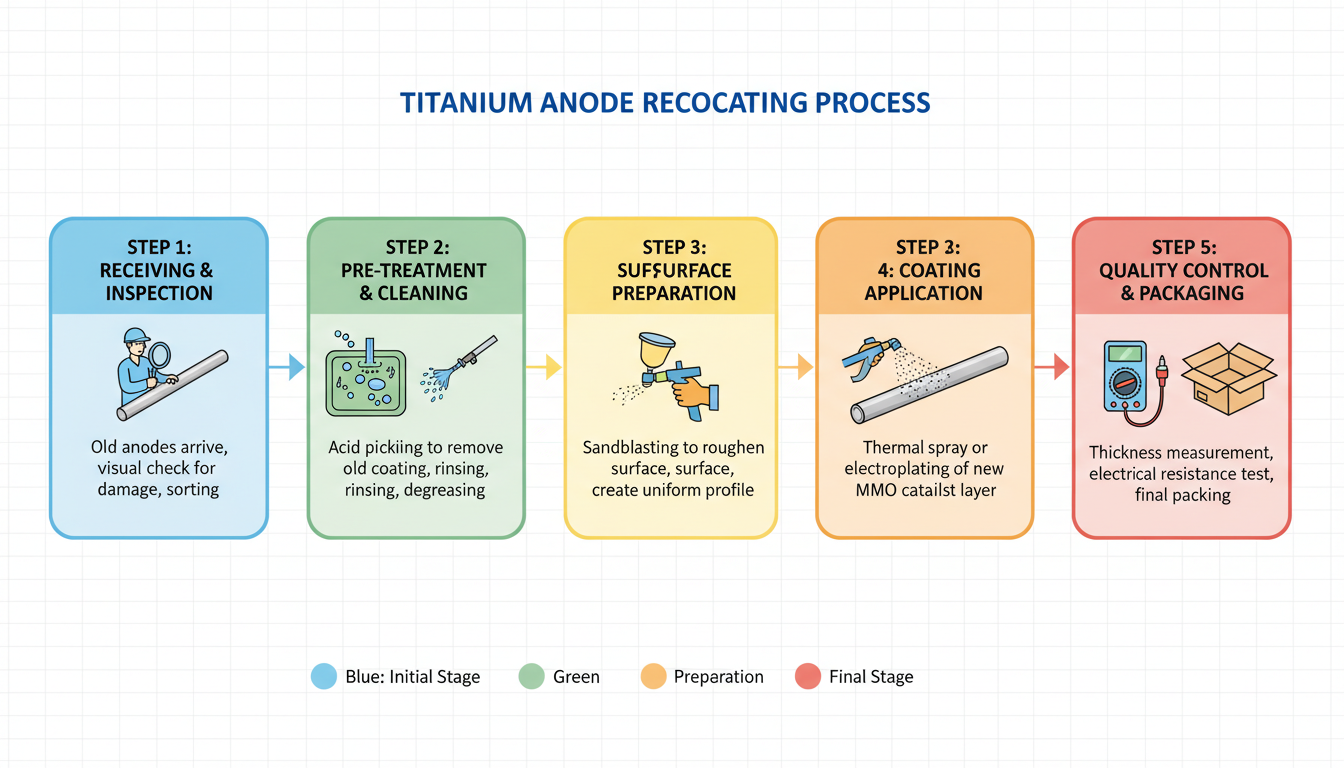
Ultimately, the decision should be data-driven. Consulting with experts in titanium fabrication and finishing, like the team at China Titanium Factory, can provide invaluable guidance. They understand the nuances of both processes and can help match the right treatment to your exact specifications.
Beyond Coloring and Anodizing: Other Metal Surface Treatments
While heat coloring and anodizing are powerful tools, the world of metal surface treatments is vast. Other techniques address different challenges:
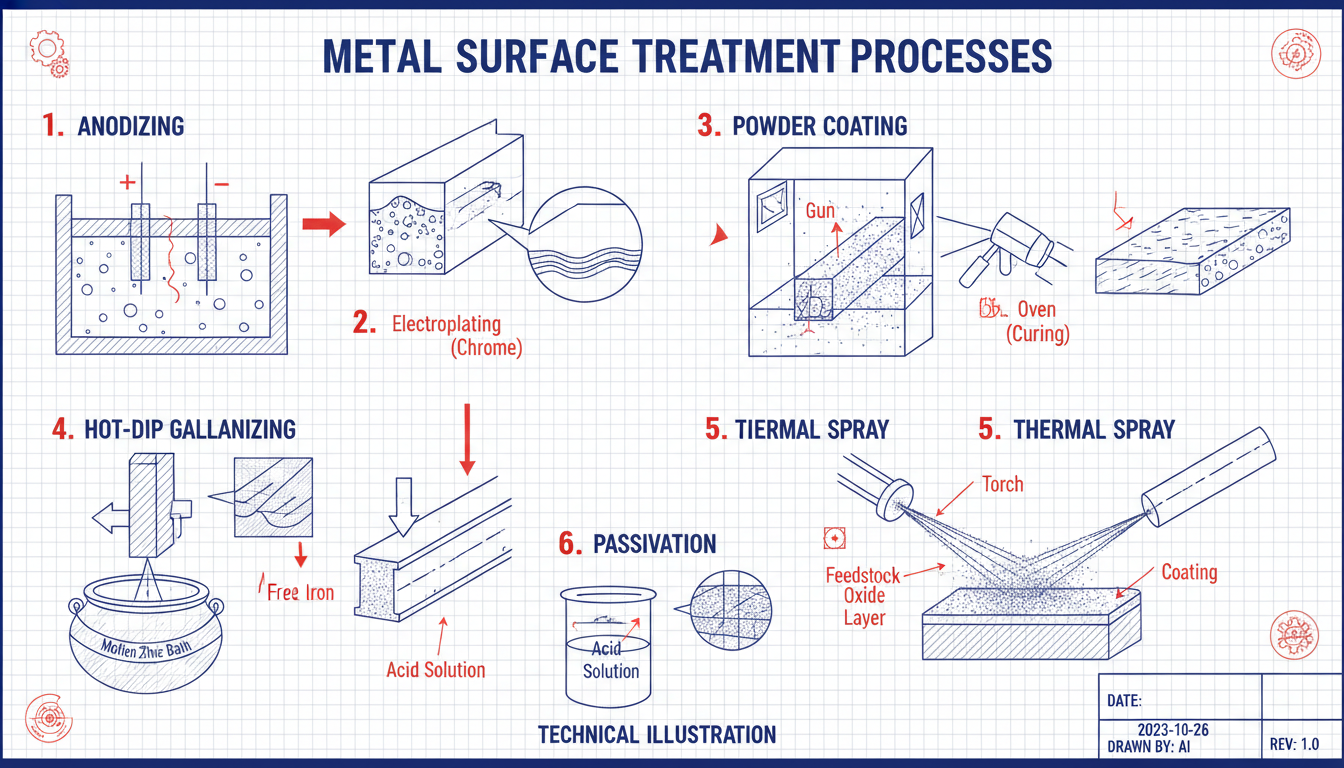
Plating: Electroplating (e.g., nickel, chrome, gold) adds a layer of a different metal for corrosion resistance, hardness, or aesthetics.
Physical Vapor Deposition (PVD): Creates thin, hard, wear-resistant coatings (e.g., TiN, CrN) by vaporizing and depositing material in a vacuum.
Chemical Conversion Coatings: Like chromate conversion on aluminum, offering moderate corrosion protection and an excellent paint base.
Thermal Spraying: Applies thick layers of various materials for extreme wear or corrosion protection.
Each method has its place, and a comprehensive understanding of your material and application will guide you to the most effective solution.
Frequently Asked Questions (FAQ)
What is the primary difference between heat coloring and electrolytic anodizing?
Heat coloring uses thermal oxidation to create a very thin, decorative oxide layer, primarily for aesthetics with minimal functional enhancement. Electrolytic anodizing uses an electrochemical process to build a thicker, denser, and more protective oxide layer, significantly improving corrosion resistance, wear resistance, and allowing for precise coloring on specific metals like aluminum and titanium.
Which metals can be heat colored versus anodized?
Heat coloring is commonly applied to steel, stainless steel, and titanium. Electrolytic anodizing is primarily effective on non-ferrous metals such as aluminum, titanium, magnesium, and niobium. Anodizing is not suitable for steel.
Does heat coloring provide corrosion resistance comparable to anodizing?
No. Heat coloring offers minimal corrosion resistance, typically requiring additional protective coatings. Anodizing, especially Type II and Type III, creates a highly durable, integral oxide layer that provides excellent, long-lasting corrosion protection, making it far superior for functional applications.
What are the cost implications of each process?
Generally, basic heat coloring can be less expensive for small batches or simple parts due to lower equipment investment. Electrolytic anodizing involves higher setup costs for specialized equipment and chemical management, but its superior performance often justifies the investment for high-value or high-volume functional components.
Can anodized parts be heat colored, or vice-versa?
Attempting to heat color an anodized part would likely degrade the anodic layer, as the high temperatures are incompatible with its structure. Conversely, anodizing a heat-colored part would typically remove or significantly alter the heat-colored oxide layer during the pre-treatment etching steps, then replace it with the anodic oxide. It's generally not advisable to combine these processes in sequence; choose one optimal treatment.
Need Expert Metal Finishing for Your Next Project?
Choosing the right surface treatment can make or break your product's performance and aesthetics. At China Titanium Factory, we specialize in advanced titanium fabrication and finishing services, including precision electrolytic anodizing. Our team of experts is ready to help you navigate complex material science challenges and deliver superior results.
Get a Quote Today