Pure titanium exhibits a remarkably high melting point of approximately 1668 °C (3034 °F).
This exceptional thermal property stems from strong metallic bonds and a stable crystal lattice structure.
Titanium's high melting point makes it indispensable for demanding applications in aerospace, medical implants, and chemical processing.
Compared to many common industrial metals like steel and aluminum, titanium's melting temperature is significantly higher.
Processing titanium requires specialized techniques due to its high reactivity at elevated temperatures.
Understanding Titanium's Melting Point: A Comprehensive Guide
Why Titanium Boasts Such a High Melting Point: The Scientific Explanation
Titanium's Melting Point Compared to Other Industrial Metals
Factors Influencing Titanium's Melting Behavior and Properties
Challenges and Innovations in Melting and Processing Titanium
Titanium, a lustrous transition metal with a silver color, holds a distinguished position among high-performance engineering materials. Its unique combination of high strength-to-weight ratio, excellent corrosion resistance, and biocompatibility makes it invaluable across numerous demanding industries. A fundamental property contributing significantly to its utility, especially in high-temperature environments, is its remarkable titanium melting point. Understanding this characteristic is crucial for engineers, material scientists, and manufacturers working with this versatile metal. This guide explores the scientific underpinnings of titanium's thermal behavior, compares it to other industrial metals, and highlights the critical applications where this property is a defining advantage.
Titanium (Ti) is a chemical element with atomic number 22. It is characterized by its low density, high strength, and exceptional resistance to corrosion, even in seawater, aqua regia, and chlorine. Discovered in 1791, titanium is the ninth most abundant element in the Earth's crust. It is never found in its pure metallic form in nature; instead, it is found in mineral deposits, primarily rutile and ilmenite. Its allotropic nature allows it to exist in two main crystalline forms: alpha (hexagonal close-packed) at lower temperatures and beta (body-centered cubic) at higher temperatures, influencing its mechanical and thermal properties.
The melting point of a substance is the temperature at which it changes state from solid to liquid. For pure titanium, this critical phase transition occurs at approximately 1668 °C (3034 °F). This value is a precise measure reflecting the energy required to overcome the interatomic forces holding the solid lattice together. The melting point is a fundamental material property, indicating the upper temperature limit for solid-state applications and dictating the processing temperatures required for casting and shaping. This high thermal threshold is a primary reason for titanium's selection in environments exposed to extreme heat.
The high melting point of titanium is attributable to specific characteristics of its atomic structure and bonding. Titanium atoms form strong metallic bonds within their crystal lattice. These bonds involve the delocalization of valence electrons, creating a "sea" of electrons that are shared among all the atoms. The strength of these metallic bonds, combined with titanium's relatively stable electron configuration (specifically, its d-orbital electrons participating in bonding), requires a substantial amount of thermal energy to break them and transition the material into a liquid state. The arrangement of atoms in its hexagonal close-packed (HCP) structure at room temperature (alpha phase) further contributes to its structural integrity and resistance to thermal agitation. According to Wikipedia, the electronic structure of titanium plays a significant role in its properties.
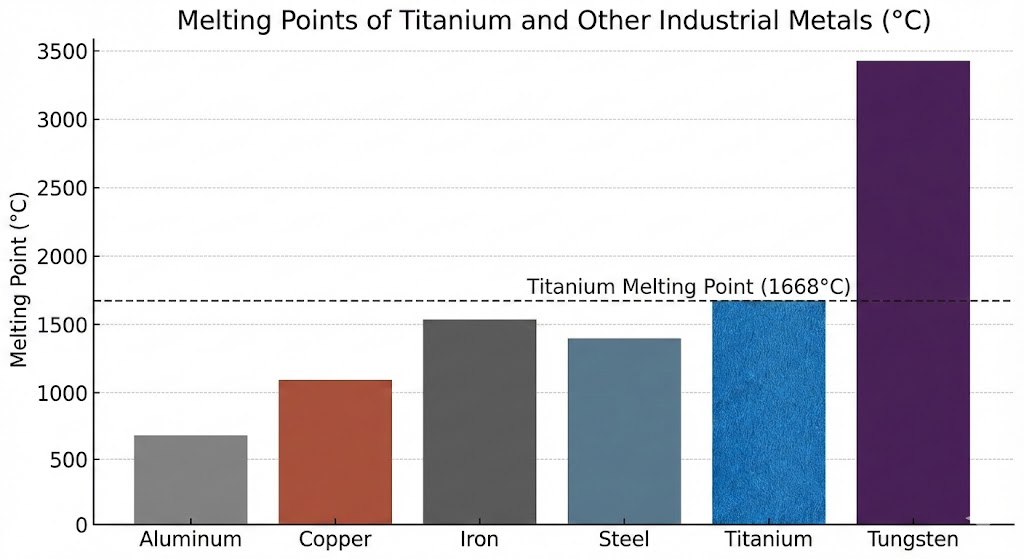
To appreciate the significance of titanium's thermal properties, it is beneficial to compare its melting point with that of other commonly used industrial metals.
| Metal | Melting Point (°C) | Melting Point (°F) |
|---|---|---|
| Titanium (Pure) | 1668 | 3034 |
| Iron (Pure) | 1538 | 2800 |
| Steel (Typical Carbon) | ~1370 - 1530 | ~2500 - 2780 |
| Aluminum (Pure) | 660.3 | 1220.5 |
| Copper (Pure) | 1085 | 1984 |
| Tungsten | 3422 | 6192 |
As the table illustrates, titanium's melting point is significantly higher than that of common structural metals like aluminum and copper. While it does not reach the extreme levels of refractory metals such as tungsten, its melting temperature is comparable to or surpasses many steels, making it a superior choice for applications demanding structural integrity at elevated temperatures where other metals would soften or deform. The National Institute of Standards and Technology (NIST) provides comprehensive data on the thermophysical properties of elements, confirming these values.
The melting behavior of titanium is not solely a fixed value; it can be influenced by several factors:
Purity Levels: Impurities, even in small amounts, can lower the melting point of titanium. Oxygen, nitrogen, hydrogen, and iron are common interstitial impurities that affect its mechanical properties and can slightly alter its thermal characteristics.
Alloying Elements: The addition of alloying elements significantly modifies titanium's properties, including its melting range. For example, the widely used Ti-6Al-4V alloy (containing 6% aluminum and 4% vanadium) has a melting range rather than a single point, typically from approximately 1604 °C to 1660 °C (2920 °F to 3020 °F). Aluminum tends to increase the melting point, while vanadium can slightly decrease it or expand the melting range. These alloys are often preferred for their enhanced strength and creep resistance at high temperatures.
Pressure: While less significant in most industrial applications, extremely high pressures can influence the melting point of materials, generally increasing it.
Understanding these influences is crucial for selecting the appropriate titanium grade or alloy for specific applications, ensuring optimal performance under various operating conditions. For specific alloy requirements, consider consulting material specification services.

The high melting point of titanium, combined with its other favorable properties, makes it indispensable in several critical industries:
Aerospace: Jet engines, airframes, and spacecraft components operate under extreme temperatures. Titanium alloys are extensively used in compressor blades, discs, and casings due to their ability to maintain strength and structural integrity at temperatures where aluminum alloys would fail. Its high melting point is vital for resisting creep and thermal fatigue in these demanding environments.
Medical Implants: While biocompatibility and corrosion resistance are primary drivers, the high melting point of titanium is advantageous in the sterilization process of surgical tools and in maintaining implant integrity during body temperature fluctuations.
Chemical Processing: Equipment like heat exchangers, pipes, and valves in chemical plants often handle corrosive substances at elevated temperatures. Titanium's resistance to corrosion and its high melting point ensure durability and safety in such aggressive environments.
Automotive and Motorsports: In high-performance vehicles, titanium is used for exhaust systems, connecting rods, and valves, where its high melting point and low density contribute to reduced weight and improved engine efficiency under intense thermal loads.
Energy Sector: Components for power generation, including those in nuclear and geothermal plants, benefit from titanium's ability to withstand high temperatures and corrosive media.
These applications underscore titanium's role as a material of choice where thermal stability and structural performance at high temperatures are paramount.
While the high melting point of titanium is a significant advantage in its applications, it presents considerable challenges during its processing. Titanium is highly reactive with oxygen, nitrogen, and carbon at elevated temperatures, leading to embrittlement if not processed correctly. This necessitates specialized melting techniques that operate in vacuum or inert atmospheres:
Vacuum Arc Remelting (VAR): This is the most common method for producing high-quality titanium ingots. It involves melting titanium electrodes in a vacuum chamber using an electric arc, which helps remove impurities and ensures homogeneity.
Electron Beam Melting (EBM): EBM uses a focused beam of electrons to melt titanium, typically in a high vacuum. This method offers precise control over the melting process and is particularly effective for removing high-vapor pressure impurities.
Plasma Arc Melting (PAM): PAM utilizes plasma torches to melt titanium, offering flexibility in terms of atmosphere and raw material forms.
Additive Manufacturing: Advancements in additive manufacturing, such as Electron Beam Powder Bed Fusion (EBPBF) and Laser Powder Bed Fusion (LPBF), allow for the creation of complex titanium parts directly from powder. These processes inherently involve localized melting and solidification in controlled atmospheres, leveraging titanium's properties while mitigating reactivity challenges.
These innovations continue to expand the possibilities for titanium fabrication, making it more accessible for diverse industrial uses.

While titanium possesses a high melting point, it is generally not classified as a refractory metal. Refractory metals, such as tungsten, molybdenum, tantalum, and niobium, are typically defined by melting points above 2000 °C (3632 °F). Titanium's melting point of 1668 °C places it just below this threshold, though its high-temperature strength is still exceptional.
The boiling point of pure titanium is approximately 3287 °C (5949 °F). This is significantly higher than its melting point, indicating a broad liquid phase range.
Yes, titanium can burn. While stable at room temperature, finely divided titanium powder or thin shavings can ignite and burn in air, producing a bright white flame. Bulk titanium is resistant to combustion under normal conditions but can burn in an atmosphere of pure oxygen or nitrogen at high temperatures. This reactivity is why processing requires inert atmospheres.
Commercially, titanium is melted using specialized techniques such as Vacuum Arc Remelting (VAR) or Electron Beam Melting (EBM). These processes are conducted in vacuum or inert gas environments to prevent titanium from reacting with atmospheric gases at high temperatures, which would lead to contamination and embrittlement.
The high melting point of titanium is a cornerstone property that underpins its utility in critical engineering applications. This thermal resilience, combined with its strength, low density, and corrosion resistance, positions titanium as an indispensable material for environments where performance under extreme conditions is non-negotiable. As industries continue to push the boundaries of temperature and stress, the demand for materials like titanium that can maintain their integrity will only grow. Continued research into titanium alloys and advanced processing techniques will further expand the applications benefiting from titanium's exceptional thermal properties, ensuring its enduring significance in materials science and engineering. The understanding of the titanium melting point remains a crucial aspect for material selection and design.
Discover how high-performance titanium materials can enhance the durability and efficiency of your most demanding applications. Partner with experts in titanium manufacturing.
Request a Consultation →