Electrode coatings are crucial for efficiency and selectivity in electrochemical processes like CER and OER.
CER coatings primarily utilize Dimensionally Stable Anodes (DSAs) based on ruthenium and iridium oxides for high chlorine selectivity.
OER coatings require robust catalysts like iridium oxide, ruthenium oxide, or non-precious metal oxides for efficient oxygen evolution in water splitting.
Selecting the optimal coating depends on operating conditions, electrolyte, current density, and desired lifetime.
Chinatiatniumfactory.com offers specialized electrode solutions tailored for both CER and OER applications.
Electrode coatings serve as critical interfaces in various electrochemical processes, fundamentally influencing reaction efficiency, selectivity, and the operational lifespan of industrial systems. These specialized layers are engineered to enhance catalytic activity, mitigate corrosion, and reduce the overpotential required for desired reactions.
The strategic application of electrode coatings directly impacts the economic viability and environmental footprint of large-scale industrial applications. This includes diverse sectors ranging from chemical production to renewable energy generation. Understanding their role is paramount for optimizing performance.
In the context of electrochemistry, two predominant anodic reactions are the Chlorine Evolution Reaction (CER) and the Oxygen Evolution Reaction (OER). Both processes demand highly specific electrode properties to achieve their respective objectives effectively.

The Chlorine Evolution Reaction (CER) and Oxygen Evolution Reaction (OER) are distinct anodic processes with differing thermodynamic and kinetic requirements. Understanding these fundamental differences is crucial for selecting appropriate electrode materials and coatings.
CER involves the oxidation of chloride ions to molecular chlorine, typically occurring in the chlor-alkali process. This reaction is highly desirable for industrial chlorine production and requires high selectivity to prevent the undesirable co-evolution of oxygen.
Definition: The Chlorine Evolution Reaction (CER) is an electrochemical process where chloride ions (Cl⁻) are oxidized at an anode to produce molecular chlorine (Cl₂), often competing with oxygen evolution.
Conversely, OER is the oxidation of water to molecular oxygen, a critical half-reaction in water electrolysis for hydrogen production and various energy conversion technologies. Minimizing overpotential for OER is a primary objective to enhance energy efficiency.
Definition: The Oxygen Evolution Reaction (OER) is an electrochemical process where water molecules are oxidized at an anode to produce molecular oxygen (O₂) and protons (H⁺) or hydroxide ions (OH⁻), depending on pH.
The standard electrode potential for CER (1.36 V vs. SHE) is relatively close to that of OER (1.23 V vs. SHE). This proximity necessitates highly selective catalysts to favor chlorine evolution over oxygen evolution when chloride is present. Source: Royal Society of Chemistry
In chloride-containing electrolytes, OER is thermodynamically more favorable than CER. However, kinetic factors and catalytic selectivity of the electrode coating are critical in ensuring that CER proceeds efficiently without significant oxygen co-evolution, which can degrade product purity and electrode performance.
Coatings for CER applications are primarily designed to achieve high selectivity for chlorine production while minimizing oxygen evolution. This is paramount in processes like the chlor-alkali industry, where chlorine purity is critical.
Dimensionally Stable Anodes (DSAs), often based on a titanium substrate coated with mixed metal oxides (MMOs), are the industry standard. These coatings typically contain ruthenium dioxide (RuO₂) and iridium dioxide (IrO₂), sometimes blended with titanium dioxide (TiO₂) or tantalum oxide (Ta₂O₅).
The ruthenium component is highly active for CER, offering excellent catalytic properties. Iridium, while also active, contributes significantly to the electrode's corrosion resistance and stability, particularly in harsh acidic and saline environments.

High Selectivity: Designed to preferentially catalyze Cl⁻ oxidation over H₂O oxidation.
Low Overpotential: Minimizes the energy input required for the reaction, leading to lower operating costs.
Corrosion Resistance: Essential for longevity in aggressive chloride-containing electrolytes.
Stability: Maintains performance over extended periods of continuous operation.
The engineering of these coatings involves precise control over composition, morphology, and crystalline structure. This ensures optimal catalytic sites and robust adhesion to the titanium substrate. Chinatitaniumfactory.com provides specialized custom coating services to meet specific industrial requirements for CER applications.
For Oxygen Evolution Reaction (OER), the primary goal is to achieve high catalytic activity and stability, particularly in challenging environments like strong acids or bases. OER is a kinetically sluggish four-electron transfer process, requiring efficient catalysts to reduce the high overpotential.
Precious metal oxides, such as iridium oxide (IrO₂) and ruthenium oxide (RuO₂), are among the most active OER catalysts, especially in acidic media. Their robust performance makes them ideal for proton exchange membrane (PEM) electrolyzers used in hydrogen production.
In alkaline environments, non-precious metal oxides and hydroxides gain prominence. Nickel-iron layered double hydroxides (NiFe-LDHs), cobalt oxides (Co₃O₄), and perovskites (e.g., BaSrCoFeOₓ) offer promising activity and often lower costs, making them suitable for alkaline electrolyzers.
Iridium Oxide (IrO₂) and Ruthenium Oxide (RuO₂): Excellent activity and stability in acidic conditions; high cost.
Nickel-Iron Layered Double Hydroxides (NiFe-LDHs): Highly active and stable in alkaline media; cost-effective.
Cobalt Oxides (Co₃O₄) and Spinels: Good activity in alkaline solutions, often used in combination with other metals.
Perovskites (e.g., LaNiO₃): Exhibiting promising OER activity and tunable electronic properties.
The choice between these materials depends heavily on the specific application, desired efficiency, and cost constraints. Research continues into developing more efficient and cost-effective OER catalysts, particularly those based on earth-abundant elements. Source: Nature Catalysis
The pH of the electrolyte significantly dictates OER catalyst selection. Iridium and ruthenium oxides are superior in acidic environments due to their stability, whereas nickel and cobalt-based materials thrive in alkaline conditions, offering excellent performance and cost advantages.
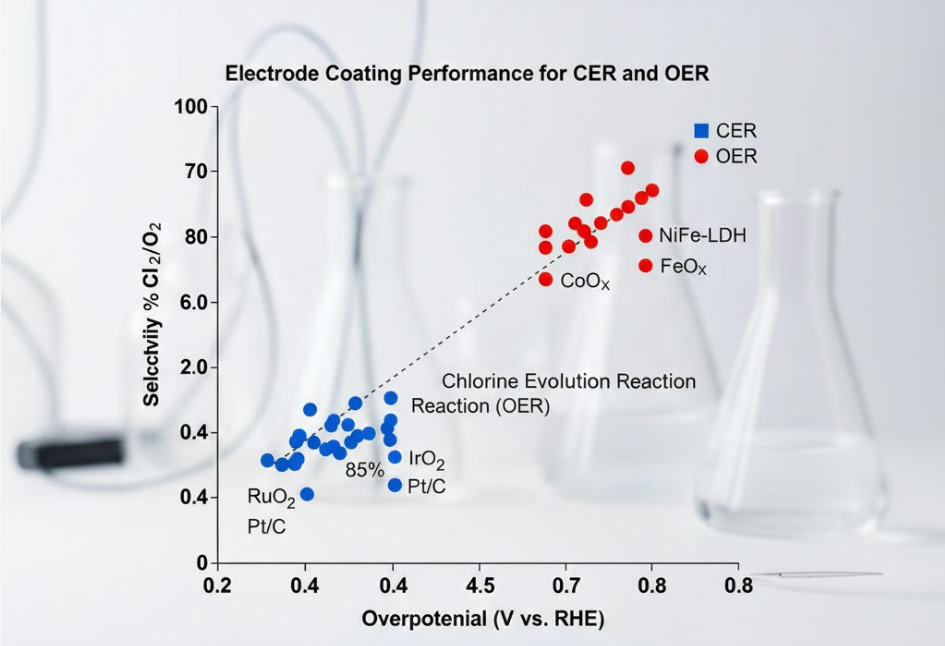
Comparing coating performance for CER and OER reveals distinct material preferences and operational priorities. While some materials, like ruthenium and iridium oxides, show activity in both, their optimal application and performance metrics differ significantly.
For CER, the focus is on achieving high chlorine selectivity and maintaining stability in highly corrosive chloride media. Ruthenium-rich MMO coatings excel here, minimizing unwanted oxygen evolution and maximizing current efficiency for chlorine production.
For OER, the primary performance indicator is low overpotential at high current densities, signifying efficient water splitting. While RuO₂ and IrO₂ are benchmark catalysts, the stability in acidic OER is paramount, often favoring IrO₂ due to its superior corrosion resistance.
| Metric | CER (e.g., RuO₂-TiO₂) | OER (Acidic, e.g., IrO₂) | OER (Alkaline, e.g., NiFe-LDH) |
|---|---|---|---|
| Primary Goal | High Cl₂ Selectivity | Low OER Overpotential | Low OER Overpotential |
| Key Catalysts | RuO₂, IrO₂, TiO₂ | IrO₂, RuO₂ | NiFe-LDH, CoOₓ, Perovskites |
| Stability Challenges | Chloride corrosion, oxygen co-evolution | Acidic dissolution, high potential degradation | Long-term alkaline stability, surface passivation |
| Cost-Effectiveness | Moderate (due to precious metals) | High (due to Ir/Ru scarcity) | Low (earth-abundant materials) |
Selecting the optimal electrode coating is a multifaceted decision influenced by several critical factors. A thorough evaluation of these parameters ensures the chosen material performs effectively and economically over its intended lifespan.
The operating conditions of the electrochemical cell are paramount. These include the electrolyte composition, pH, temperature, and current density. Each of these variables can drastically alter the stability and catalytic activity of a given coating material.
Desired coating lifetime and overall cost analysis also play significant roles. While precious metal oxides often offer superior performance, their higher initial cost necessitates a detailed assessment of their total cost of ownership versus less expensive, but potentially less durable, alternatives.

Electrolyte Composition: Presence of chlorides, sulfates, or other ions significantly impacts reaction pathways and corrosion.
pH and Temperature: Affect catalyst stability, solubility, and reaction kinetics.
Current Density: Dictates the required catalytic activity and influences electrode degradation rates.
Mechanical Stability: Resistance to erosion and delamination under operational stress.
Environmental Impact: Consideration of material sourcing, toxicity, and recyclability.
Manufacturers like chinatitaniumfactory.com often provide detailed specifications and can offer consultation to tailor coatings for specific industrial needs. This ensures optimal performance and longevity for the given application.
The practical implementation of optimized electrode coatings for CER and OER has driven significant advancements across various industrial sectors. These applications demonstrate the tangible benefits of selecting the correct coating for specific electrochemical processes.
In the chlor-alkali industry, the transition from graphite anodes to DSA electrodes coated with RuO₂-TiO₂ revolutionized chlorine and caustic soda production. This change drastically reduced energy consumption, increased cell efficiency, and extended anode lifetime from months to several years.
For water electrolysis, particularly for green hydrogen production, advanced OER coatings are indispensable. PEM electrolyzers, utilizing IrO₂-coated anodes, achieve high current densities and efficiency, crucial for renewable energy integration. Alkaline electrolyzers increasingly employ NiFe-LDH coatings, offering a cost-effective alternative for large-scale hydrogen generation. Source: ScienceDirect - Renewable and Sustainable Energy Reviews

Energy Efficiency: Reduced overpotential directly translates to lower electricity consumption per unit of product.
Product Purity: Enhanced selectivity minimizes unwanted byproducts, reducing purification costs.
Operational Lifespan: Corrosion-resistant coatings extend electrode durability, reducing maintenance and replacement cycles.
Cost Reduction: Long-term savings from improved efficiency and extended equipment life.
These case studies underscore the strategic importance of tailoring electrode coatings to specific reaction environments. For further insights into custom solutions, contact Chinatitaniumfactory.com to discuss your project needs.
The field of electrode coating technology is continuously evolving, driven by the demand for higher efficiency, lower costs, and enhanced sustainability in electrochemical processes. Emerging trends focus on novel materials, advanced fabrication techniques, and data-driven design.
Research is actively exploring nanostructured catalysts to maximize active surface area and improve intrinsic activity. This includes nanoparticles, nanowires, and porous structures that can significantly boost performance with minimal material usage.
The development of non-precious metal catalysts for OER, particularly in acidic media, remains a significant challenge and a key area of investigation. Efforts are concentrated on creating earth-abundant alternatives that can match the performance of iridium and ruthenium.
High-Entropy Alloys (HEAs): Exploring multi-metallic systems for synergistic catalytic effects and improved stability.
Machine Learning and AI: Utilizing computational methods for accelerated material discovery and optimization of coating compositions and structures.
Sustainable Manufacturing: Developing greener synthesis routes for coatings, reducing hazardous waste, and improving material recyclability.
In-situ and Operando Characterization: Gaining deeper understanding of reaction mechanisms and degradation pathways under actual operating conditions.
These advancements promise to deliver a new generation of electrode coatings that are not only more efficient and durable but also more environmentally friendly and economically viable. The Chinatitaniumfactory.com blog often features articles on these cutting-edge developments in electrocatalysis.
The market for industrial electrode coatings is characterized by specialized manufacturers offering a range of standard and customized solutions for CER and OER. These suppliers focus on delivering high-performance, durable, and cost-effective electrode materials.
Commercial electrode coatings are typically supplied as finished anodes, often on a titanium substrate, or as coating formulations for in-house application. Product specifications include catalyst composition, coating thickness, expected lifetime, and performance under specific operating conditions.
Key considerations for procurement involve assessing the supplier's technical expertise, their ability to provide custom solutions, and their track record in delivering reliable products that meet stringent industrial standards. Technical support and after-sales service are also crucial.

Technical Expertise: Look for suppliers with deep knowledge in electrochemistry and materials science.
Customization Capabilities: Ability to tailor coating compositions and geometries for unique applications.
Quality Assurance: Adherence to international quality standards and robust testing protocols.
Reliability and Lead Times: Consistent product delivery and responsive service.
Chinatitaniumfactory.com is a prominent provider of custom titanium electrodes and coatings, specializing in solutions for both CER and OER applications. The company offers a range of high-performance products designed for demanding industrial environments.
The primary difference lies in selectivity and stability. CER coatings require high selectivity for chlorine production over oxygen evolution and robust stability in highly corrosive chloride environments. OER coatings prioritize low overpotential and high activity for water oxidation, with stability tailored to either acidic (e.g., PEM electrolyzers) or alkaline (e.g., alkaline electrolyzers) conditions.
DSAs, typically titanium substrates coated with mixed metal oxides like RuO₂-TiO₂, offer exceptional catalytic activity, high selectivity for chlorine, and superior corrosion resistance compared to older graphite electrodes. This combination leads to significantly lower energy consumption, extended operational life, and reduced maintenance in chlor-alkali processes.
While some materials like RuO₂ and IrO₂ exhibit activity for both reactions, their optimal performance and application conditions differ. Coatings are typically specialized to maximize efficiency and selectivity for one reaction over the other. Using a CER-optimized coating for OER, or vice-versa, would likely result in suboptimal performance, higher energy consumption, or reduced lifespan.
Several factors influence coating lifespan, including current density, electrolyte composition, temperature, pH, and the presence of impurities. Higher current densities, aggressive electrolytes, and elevated temperatures can accelerate coating degradation. Proper selection, maintenance, and operating within design parameters are crucial for maximizing electrode longevity.
Non-precious metal oxides (e.g., NiFe-LDHs, CoOₓ) are significantly more cost-effective and show excellent OER activity and stability, particularly in alkaline environments. However, they generally do not match the performance or stability of precious metal oxides (IrO₂, RuO₂) in highly acidic conditions, which are critical for certain applications like PEM electrolyzers.
The selection of an appropriate electrode coating for either Chlorine Evolution Reaction (CER) or Oxygen Evolution Reaction (OER) is a strategic decision with profound implications for industrial efficiency, cost-effectiveness, and environmental sustainability. A deep understanding of reaction mechanisms, material properties, and operational parameters is essential.
For CER, the emphasis remains on highly selective and corrosion-resistant ruthenium- and iridium-based mixed metal oxide coatings on titanium substrates. These materials ensure high purity chlorine production while minimizing undesirable oxygen co-evolution.
For OER, the choice is more diverse, ranging from precious metal oxides for acidic environments to earth-abundant nickel and cobalt-based materials for alkaline systems. The goal is consistently to reduce overpotential and enhance long-term stability for efficient water splitting.
Ultimately, making the optimal choice requires a comprehensive evaluation of application-specific needs, including electrolyte chemistry, temperature, current density, desired lifespan, and budget constraints. Consulting with experienced material suppliers and electrochemists is highly recommended to tailor solutions precisely.
Ensure your electrochemical processes achieve maximum efficiency and longevity. Partner with Chinatitaniumfactory.com for custom-engineered electrode coatings tailored to your exact CER or OER application.
Request a Consultation Today