Understanding Titanium Anodizing and the Critical Role of Electrolytes
Achieving a specific, high-quality finish on titanium often feels like a shot in the dark for many manufacturers and designers. Inconsistent colors, uneven surfaces, or poor durability plague projects, leading to wasted time and materials. The culprit? Often, it's a misunderstanding of the core of the process: the electrolyte solution.
Titanium anodizing is more than just a surface treatment. It's an electrochemical process that grows a protective, often colorful, oxide layer on the titanium substrate. This layer doesn't just look good; it enhances corrosion resistance, biocompatibility, and wear properties. Think of it as giving titanium a superhero suit.
The magic happens when titanium, immersed in an electrolyte solution, is subjected to an electric current. The electrolyte acts as the medium, facilitating the controlled oxidation of the titanium surface. Without the right electrolyte, achieving a vibrant purple, a subtle bronze, or even a robust, clear protective layer is simply not possible.
The choice of electrolyte is paramount. It dictates the oxide layer's thickness, density, and ultimately, its optical properties – which translate directly into the observed color. A well-chosen electrolyte is the difference between a dull, inconsistent finish and a stunning, durable one that meets stringent industrial standards.

Exploring the Spectrum: Common Electrolyte Solutions for Titanium Anodizing
Not all electrolytes are created equal. Different solutions yield distinct results, each with its own set of chemical properties and ideal applications.
Here's a rundown of the workhorses in the titanium anodizing world:
Trisodium Phosphate (TSP)
TSP is a go-to for many beginners and professionals alike. It's alkaline, mild, and relatively safe to handle. A typical concentration might be 5-10% by weight in distilled water.
Trisodium Phosphate (TSP): An inorganic compound with the formula Na₃PO₄. It is a white, granular or crystalline solid, highly soluble in water, producing an alkaline solution. Commonly used as a cleaning agent, degreaser, and in anodizing due to its mild electrolytic properties.
TSP generally produces a broad spectrum of colors, particularly vibrant blues and purples, making it popular for decorative finishes. It's forgiving, offering good consistency without aggressive etching.
Sulfuric Acid (H₂SO₄)
A strong mineral acid, sulfuric acid is less common for color anodizing titanium but finds its niche in Type II (sulfuric acid) anodizing for aluminum, and sometimes for specific titanium applications where a thick, hard, and wear-resistant oxide layer is prioritized over vibrant color. Concentrations vary significantly, from 5% to 20% or more, requiring careful handling.
Phosphoric Acid (H₃PO₄)
Phosphoric acid is often used for Type III (hardcoat) anodizing on aluminum, creating very dense, abrasion-resistant layers. For titanium, it can produce a range of colors, similar to TSP, but with potentially better control over specific hues due to its more aggressive nature. Typical concentrations are in the 10-15% range. It requires more caution than TSP.
Borax (Sodium Tetraborate)
Similar to TSP, borax is a mild alkaline salt. It's often used in dilute solutions (e.g., 2-5%) and is known for producing clear, consistent oxide layers and a good range of colors. Its mildness makes it a safer option for hobbyists or educational settings.
Sodium Hydroxide (NaOH)
Also known as lye or caustic soda, NaOH is a very strong alkali. While it can be used for etching titanium, it's generally not recommended as the primary electrolyte for color anodizing due to its aggressive nature, which can lead to excessive etching and a less uniform oxide layer. It demands extreme caution in handling.
Each of these electrolytes has its quirks. Understanding them is half the battle in achieving your desired custom titanium fabrication finish.
Choosing Your Medium: A Comparative Analysis of Electrolyte Solutions
Picking the right electrolyte isn't just about what's available; it's about matching the solution to your project's demands. Cost, safety, and the desired aesthetic all play a role. Let's break down the options.

The table below offers a concise comparison, helping you weigh the pros and cons for your specific application.
| Electrolyte | Cost-Effectiveness | Safety Considerations | Achievable Colors/Finish | Application Suitability |
|---|---|---|---|---|
| Trisodium Phosphate (TSP) | Low | Relatively low risk (mild alkali) | Broad spectrum, vibrant (esp. blues/purples) | Decorative items, jewelry, hobby projects |
| Sulfuric Acid | Low to Medium | High risk (strong acid, corrosive) | Limited color range; focus on layer hardness | Industrial components, wear resistance (less for color) |
| Phosphoric Acid | Medium | Medium risk (strong acid, corrosive) | Good color range, often more controllable hues | Medical implants, aerospace (where precise control is key) |
| Borax | Low | Low risk (mild alkali, common household item) | Clear, consistent layers; good color spectrum | Hobbyists, educational use, fine art |
For applications like medical implants, where biocompatibility is paramount, a solution like phosphoric acid might be favored for its control over the oxide layer's properties. For decorative pieces, TSP or borax offer cost-effective and vibrant results. Always consider the end-use of your high-quality titanium products when making your choice.
Beyond the Basics: How Electrolyte Concentration and Purity Impact Your Anodizing
The electrolyte's identity is just one piece of the puzzle. Its concentration and purity are absolute game-changers for consistent results. Think of it like baking: too much or too little of an ingredient, or a stale one, can ruin the whole batch.
Concentration: The Hidden Lever of Color and Consistency
Slight variations in electrolyte concentration can drastically alter the final color and the quality of the oxide layer. A higher concentration might lead to faster film growth but could also result in a rougher, more porous surface. Conversely, a too-dilute solution might produce a very thin film, or no color at all, even at high voltages.
For example, a 5% TSP solution will yield different results than a 10% solution, even with identical voltage settings. This is because the ionic conductivity changes, affecting the rate of oxidation. Precision is key. Always use distilled or deionized water for preparation to ensure a consistent baseline.
Purity: The Unseen Guard of Surface Quality
Impurities are the bane of anodizing. Even trace amounts of contaminants—like dissolved metals from electrodes, tap water minerals, or residues from inadequate part cleaning—can cause defects. We're talking about pitting, uneven color, hazy spots, or even complete failure of the oxide layer to form properly.
Regular maintenance is non-negotiable. Filter your electrolyte to remove suspended particles. Monitor its pH and conductivity, replenishing components as they're consumed or diluted. Over time, the electrolyte degrades, becoming less effective. Knowing when to replace it entirely is crucial for maintaining rigorous quality control.
This meticulous attention to detail is what sets professional anodizing apart. It ensures every piece of titanium materials gets the finish it deserves.
Safe Handling & Responsible Disposal: Electrolyte Best Practices
Working with chemicals, even mild ones, demands respect and adherence to safety protocols. A moment of carelessness can lead to serious injury or environmental harm. Safety isn't an option; it's fundamental.
Chemical Safety: Your Personal Protective Equipment (PPE)
Always consult the Safety Data Sheet (SDS) for any chemical you use. This document is your bible for safe handling, storage, and emergency procedures. For electrolytes like sulfuric or phosphoric acid, this is especially critical.
Essential PPE includes:
Eye Protection: Chemical splash goggles are a must.
Hand Protection: Chemical-resistant gloves (nitrile or neoprene are often suitable).
Body Protection: Lab coat or apron to protect clothing and skin.
Respiratory Protection: Ensure adequate ventilation or use a respirator if fumes are a concern.
Work in a well-ventilated area, preferably under a fume hood. Have an eyewash station and emergency shower readily available. Know how to react to spills: contain, neutralize (if appropriate and safe), and absorb.
Responsible Disposal: Protecting Our Planet
Never pour used electrolyte solutions down the drain without proper treatment. Electrolytes can be hazardous waste, containing heavy metals (from dissolved titanium or electrodes) and acidic/alkaline components that harm ecosystems.
Disposal methods vary by local regulations. Generally, you'll need to:
Neutralize: Adjust the pH to a neutral range (6-8) using appropriate acids or bases.
Precipitate Metals: If heavy metals are present, specific chemicals can precipitate them out, allowing for filtration.
Consult Waste Management: Contact a licensed hazardous waste disposal company. They can handle the collection and proper treatment of your spent solutions.
Proper waste management isn't just a legal requirement; it's a commitment to environmental stewardship. For more detailed guidelines on chemical safety and waste disposal, resources like the Occupational Safety and Health Administration (OSHA) and local environmental protection agencies are invaluable.
From Prep to Polish: A Step-by-Step Guide to Titanium Anodizing
Anodizing isn't just about dipping metal into a bath. It's a precise sequence of steps, each critical for a flawless finish. Skip a step, and you risk compromising the entire job.
Surface Preparation: The Foundation of Flawless Finish
This is where many projects go sideways. Any grease, oil, fingerprints, or embedded contaminants will prevent uniform oxide growth.
Cleaning: Start with a thorough degreasing. Ultrasonic cleaning in an alkaline solution (like a mild detergent or specialized degreaser) works wonders. Rinse completely with distilled water.
Etching (Optional but Recommended): A mild etch can remove surface impurities and create a uniform surface texture for better oxide adhesion. Solutions like hydrofluoric acid (highly hazardous, professional use only) or a mixture of nitric and hydrofluoric acid are used. For safer, less aggressive methods, a mild alkaline etch can also prepare the surface.
Rinsing: Rinse, rinse, and rinse again with distilled or deionized water after each step. Contamination carried from one bath to the next spells trouble.
Equipment Checklist: What You'll Need
Gather your tools before you begin.
Power Supply: A DC power supply with adjustable voltage and current limiting. This is non-negotiable.
Anodizing Tank: Non-conductive, acid-resistant plastic (HDPE, polypropylene).
Cathode: A piece of inert conductive material, typically stainless steel, carbon, or lead. Larger than your anode (titanium part).
Anode: Your titanium workpiece, securely fixtured.
Electrolyte Solution: Prepared to the correct concentration.
Agitation: A stir bar or pump can help maintain solution uniformity.
Thermometer: To monitor electrolyte temperature.
Safety Gear: As discussed.
The Anodizing Procedure: Step by Step
With everything prepared, the actual anodizing is straightforward:
Submerge: Carefully submerge the cleaned titanium part (anode) and the cathode into the electrolyte solution. Ensure they do not touch.
Connect Power: Connect the positive lead of the power supply to the titanium part and the negative lead to the cathode.
Apply Voltage: Slowly increase the voltage to the desired level. Watch the current; it will typically spike and then drop as the oxide layer forms. The voltage directly correlates to the final color.
Hold Voltage: Maintain the target voltage until the current drops to a very low, stable level. This indicates the oxide layer has reached its maximum thickness for that voltage. This usually takes a few seconds to a few minutes.
Rinse: Carefully remove the anodized part from the bath and rinse thoroughly with distilled water.
Dry: Dry completely.
For complex geometries or high-volume production, specialized titanium anodizing services can ensure optimal results.
The Art of Color: Mastering Voltage and Techniques for Vibrant Titanium
Titanium's ability to display a rainbow of colors through anodizing is truly captivating. It’s not magic; it’s optics. The oxide layer created during anodizing acts as a thin-film interference filter. Light hitting the surface reflects off both the top of the oxide layer and the titanium underneath. When these reflected light waves interact, certain wavelengths cancel out or reinforce, producing the colors we see.
The Direct Link: Voltage and Color
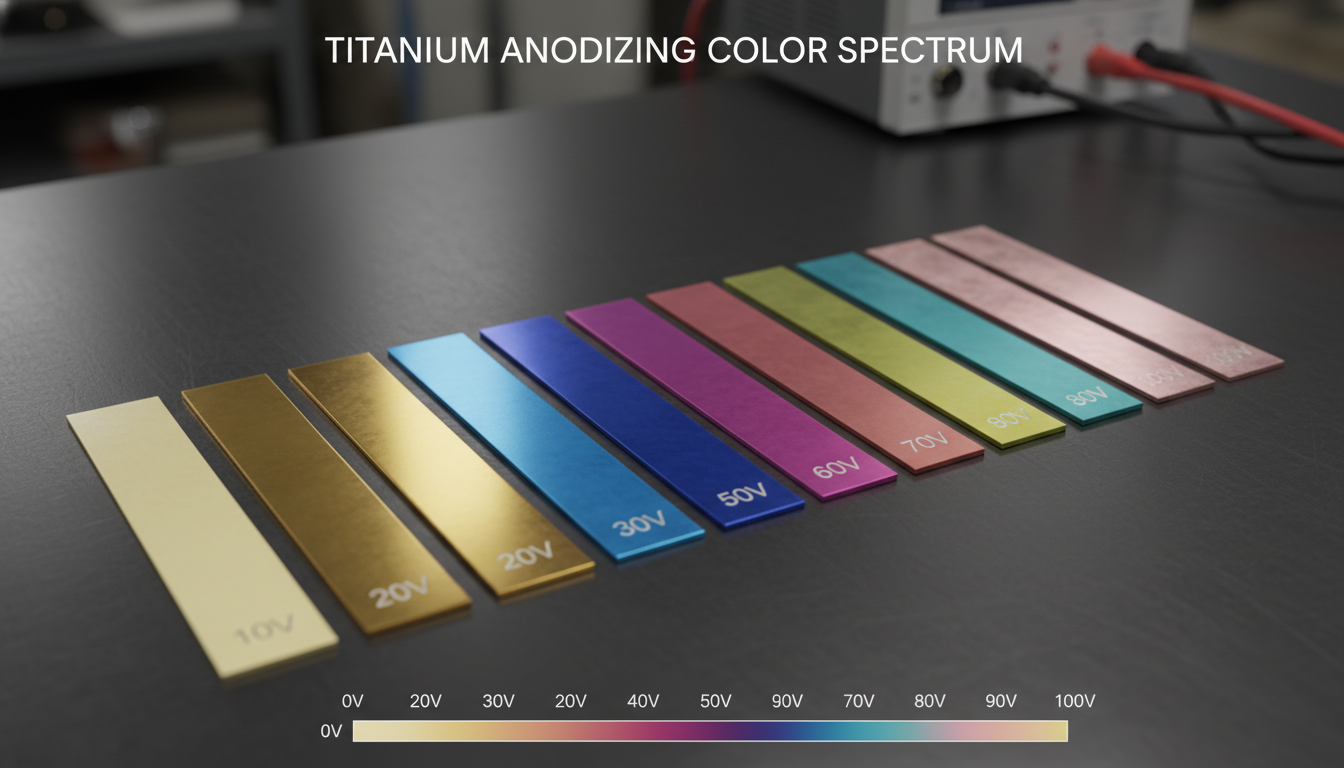
The thickness of this oxide layer is directly proportional to the applied voltage. More voltage means a thicker oxide layer. A thicker layer means different light wavelengths interfere, resulting in a different color. This relationship is remarkably predictable.
Here’s a general guide for titanium (though exact voltages can vary slightly based on alloy and electrolyte):
10-20V: Light Bronze/Brown
20-30V: Blue/Dark Blue
30-40V: Yellow/Gold
40-50V: Pink/Purple
50-60V: Teal/Green
60-70V: Light Green/Yellow-Green
70-80V: Rose/Magenta
80-90V: Light Blue/Indigo
90-100V: Silver/Gray (thicker interference colors often cycle back to subtle hues)
A stable power supply is your best friend here. Small voltage fluctuations lead to inconsistent colors. Consistency is key.
Techniques for Multi-Color and Specific Finishes
Want more than one color on a single piece? It's achievable with careful masking or sequential anodizing:
Masking: Use electrical tape, nail polish, or specialized resist paints to mask off areas you want to remain a certain color. Anodize at a lower voltage, remove the mask, then re-mask different areas, and anodize at a higher voltage. The previously anodized areas will simply get a thicker, darker oxide layer, thus changing to the next color in the spectrum.
Gradient Anodizing: Slowly immerse or withdraw the titanium piece from the electrolyte while the voltage is applied, creating a continuous color gradient.
Heat Anodizing: While not strictly electrochemical, controlled heating can also produce colors on titanium. This process is less precise for color control but can create unique, earthy tones.

Mastering these techniques transforms simple titanium parts into works of art. It’s an interplay of science and artistic vision.
Solving the Snags: Troubleshooting Common Anodizing Problems
Even with careful planning, things can go awry. Anodizing isn't always a smooth ride. Knowing how to diagnose and fix common issues saves time, material, and frustration. Most problems can be traced back to preparation, the electrolyte, or the power supply.
Uneven Color or Splotches
This is a frequent complaint.
Problem: Color is not uniform across the surface; some areas are lighter or darker, or splotchy.
Solution:
Inadequate Cleaning: The most common culprit. Re-clean the part thoroughly, focusing on degreasing.
Poor Electrical Contact: Ensure the anode clip makes solid contact with the titanium. A loose connection causes resistance and uneven current distribution.
Electrolyte Issues: Low concentration, depleted electrolyte, or uneven temperature can cause this. Agitate the solution, check concentration, or consider replacing it.
Gas Bubbles: Hydrogen bubbles can cling to the part, insulating areas. Gently agitate the part during anodizing to dislodge them.
Pitting or Rough Surface
Nobody wants a pitted finish.
Problem: Small holes or a generally rough texture appears on the anodized surface.
Solution:
Aggressive Etching: If you etched the part, the solution might be too strong or the immersion time too long. Reduce etching time or dilute the etch.
Contaminated Electrolyte: Impurities can aggressively attack the titanium. Filter or replace the electrolyte.
Excessive Current Density: Too much current for the surface area can cause localized heating and pitting. Reduce voltage or increase cathode surface area.
Poor Adhesion of Oxide Layer or Inconsistent Results
The oxide layer should be tough. If it's flaky or inconsistent:
Problem: The oxide layer rubs off easily, or color is inconsistent between batches.
Solution:
Insufficient Surface Preparation: Again, cleaning is paramount. Residues prevent proper adhesion.
Wrong Electrolyte: The electrolyte might not be suitable for forming a dense, adherent layer on your specific titanium alloy.
Voltage Fluctuations: An unstable power supply can lead to uneven layer growth. Invest in a quality, regulated DC power supply.
Electrolyte Degradation: Over time, the electrolyte loses effectiveness. Replace it.
Troubleshooting is an iterative process. Address one variable at a time. Keep detailed notes on voltage, time, temperature, and electrolyte composition. This data is gold for consistency.
Pushing Boundaries: Advanced Electrolyte Formulations and Future Innovations
While the basic electrolytes serve most needs, researchers and specialty manufacturers constantly explore new formulations. The goal? To achieve unique properties, enhance performance, or simplify the process.
Specialized Formulations for Unique Finishes
Beyond the standard phosphoric or TSP, some advanced electrolytes incorporate organic acids or specific additives. These might be designed to:
Enhance Corrosion Resistance: Certain additives can promote a denser, more impervious oxide layer, crucial for demanding environments like marine or chemical processing.
Create Superhydrophobic Surfaces: By controlling surface morphology at a nanoscale, specialized electrolytes can yield surfaces that repel water more effectively.
Achieve Novel Colors or Textures: Research into electrolytes with specific chelating agents or buffering systems can unlock colors outside the typical voltage spectrum or create unique iridescent effects.
One area of active research involves plasma electrolytic oxidation (PEO), also known as micro-arc oxidation (MAO). This process uses higher voltages and specialized electrolytes to create ceramic-like, extremely hard, and wear-resistant coatings, often with better adhesion than conventional anodizing. This is a robust solution for industrial components facing extreme conditions.
Emerging Trends and Research
The future of titanium anodizing electrolytes is moving towards:
Environmentally Friendlier Options: Reducing or eliminating hazardous chemicals, focusing on biodegradable or recyclable electrolytes.
Nanostructured Coatings: Developing electrolytes that can precisely control the nanostructure of the oxide layer, leading to enhanced functionality (e.g., photocatalysis, drug delivery).
In-situ Monitoring and Control: Advanced sensor technology to monitor electrolyte health in real-time, allowing for dynamic adjustments and predictive maintenance.
For example, studies have explored the use of ionic liquids as novel electrolytes for anodizing, offering advantages in terms of stability and reduced environmental impact (Source: Journal of Physical Chemistry C). These innovations promise even greater versatility and performance for anodized titanium in the years to come. Staying abreast of these developments is essential for pushing the boundaries of material science.
Beyond Aesthetics: Diverse Applications of Anodized Titanium
While the vibrant colors of anodized titanium are undeniably appealing, its functional benefits are what truly make it a superstar material across countless industries. The enhanced properties of the oxide layer open doors to critical applications where performance cannot be compromised.
Medical Implants: The Biocompatible Edge
Titanium is already highly biocompatible, meaning it's well-tolerated by the human body. Anodizing takes this a step further. The controlled oxide layer improves osseointegration – the process where bone grows directly onto the implant surface – which is vital for long-term success of dental and orthopedic implants. It also increases corrosion resistance within the body's harsh environment. The sterile, inert surface is perfect for internal use. For instance, ASTM F136 specifies surgical implant applications for titanium alloys, where anodizing often plays a role.
Aerospace Components: Durability in the Extreme
In aerospace, every gram and every micron of protection counts. Anodized titanium offers superior corrosion resistance against harsh atmospheric conditions, jet fuels, and de-icing fluids. It also provides a hard, wear-resistant surface, crucial for components subject to friction or erosion. This extended lifespan and reduced maintenance are invaluable in aviation and space exploration.
Decorative Finishes and Other Industrial Uses
Beyond the high-stakes fields, anodized titanium shines in:
Jewelry & Art: The vibrant, permanent colors make titanium a popular choice for unique, lightweight jewelry and sculptural art.
Consumer Electronics: Aesthetic appeal, scratch resistance, and often, electromagnetic shielding.
Sporting Goods: From bicycle frames to knife handles, improved durability and a custom look.
Architectural Elements: Long-lasting, weather-resistant facades with distinctive hues.
The versatility of anodized titanium, from life-saving medical devices to eye-catching consumer goods, truly underscores the importance of mastering the anodizing process and, by extension, the critical role of electrolyte solutions.
Your Questions Answered: Frequently Asked Questions About Electrolytes for Titanium Anodizing
What is the best electrolyte solution for achieving vibrant colors on titanium?
For vibrant colors, especially across a broad spectrum, a dilute solution of Trisodium Phosphate (TSP) or Borax (Sodium Tetraborate) is generally recommended. These mild alkaline solutions offer good control over oxide layer growth, producing consistent and bright interference colors. Phosphoric acid can also yield excellent results with precise control.
How does electrolyte concentration affect the anodizing process and final color?
Electrolyte concentration significantly impacts the process. A higher concentration typically increases conductivity, allowing for faster film growth and potentially requiring lower voltages to achieve certain colors. However, too high a concentration can lead to aggressive etching, a less uniform oxide layer, or a duller finish. Too dilute, and the film may grow too slowly or be too thin. Consistent concentration, usually 5-10% for TSP/Borax, is crucial for repeatable results.
Is it safe to reuse anodizing electrolytes, and how often should they be replaced?
You can reuse electrolytes, but their effectiveness diminishes over time. Contaminants (like dissolved titanium ions from the process) build up, and the active components deplete. This leads to inconsistent colors, pitting, or poor adhesion. Regularly filter your solution and monitor its pH and conductivity. For hobbyists, replacing the solution every few months or after significant use is a good rule of thumb. In industrial settings, continuous filtration, replenishment, and periodic complete replacement are standard practice based on throughput and quality requirements.
What are the environmental concerns and proper disposal methods for electrolyte waste?
Electrolyte waste poses environmental concerns due to its pH (being acidic or alkaline) and potential heavy metal content (dissolved titanium). Never dispose of it down the drain. Proper disposal involves neutralizing the solution to a safe pH range (6-8) and, if necessary, precipitating out heavy metals. Always consult local environmental regulations and work with a licensed hazardous waste disposal company. Responsible disposal protects ecosystems and adheres to legal mandates.
Can different titanium alloys be anodized using the same electrolyte and process?
Yes, most common titanium alloys (e.g., Grade 2, Grade 5/Ti-6Al-4V) can be anodized using the same general electrolytes and processes. However, slight variations in the resulting colors or the optimal voltage range might occur due to differences in alloy composition. Always perform test anodizing on scrap pieces of your specific alloy to fine-tune your parameters and ensure desired results. Minor adjustments to voltage or electrolyte concentration might be necessary for perfect color matching.