Mastering the Ag/AgCl Reference Electrode: Stability in Every Measurement
Precision in electrochemistry isn't about the flashy spikes on a graph. It is about the quiet, unmoving baseline. In any electrochemical cell, the Ag/AgCl reference electrode acts as the "sacrificial lamb." It takes the brunt of the potential fluctuations so your working electrode doesn't have to. Without this anchor, your data is just noise.
At China Titanium Factory, we define the "Sacrificial Lamb Principle" as the mandatory use of a high-quality reference point to isolate the variables of your reaction. If your reference drifts, your entire experiment is compromised. You need a constant potential you can bet your PhD on.

What is an Ag/AgCl Reference Electrode? (The Lab's Reliable Anchor)
The Ag/AgCl electrode is a silver wire coated with a layer of solid silver chloride, immersed in a solution of potassium chloride (KCl). It is a "half-cell" that maintains a fixed potential. Because the reaction involves both a solid metal and its sparingly soluble salt, the potential remains incredibly stable even if a small amount of current passes through it.
"The Ag/AgCl reference electrode is a non-polarizable electrode consisting of a silver metal wire (Ag) in contact with a thin layer of silver chloride (AgCl), which is in equilibrium with a chloride ion solution."
This setup is preferred over the Standard Hydrogen Electrode (SHE) for one simple reason: convenience. You don't need a tank of hydrogen gas and a platinum flame to get accurate results. It is the practical choice for 99% of researchers.
Technical Specifications: Potential, Temperature, and Electrolytes
The potential of an Ag/AgCl electrode is not a single number. It shifts based on the concentration of the internal filling solution. Most labs use 3M KCl or Saturated KCl. Saturated solutions are easier to maintain but are highly sensitive to temperature fluctuations.
| Electrolyte Concentration | Potential (mV) | Temp. Coefficient (mV/°C) |
|---|---|---|
| 3.0 M KCl | +210 | -0.65 |
| 3.5 M KCl | +205 | -0.73 |
| Saturated KCl | +197 | -1.01 |
When performing high-precision electrochemical testing, always record your temperature. A 5-degree shift can move your potential by 5mV—enough to ruin a delicate CV curve.
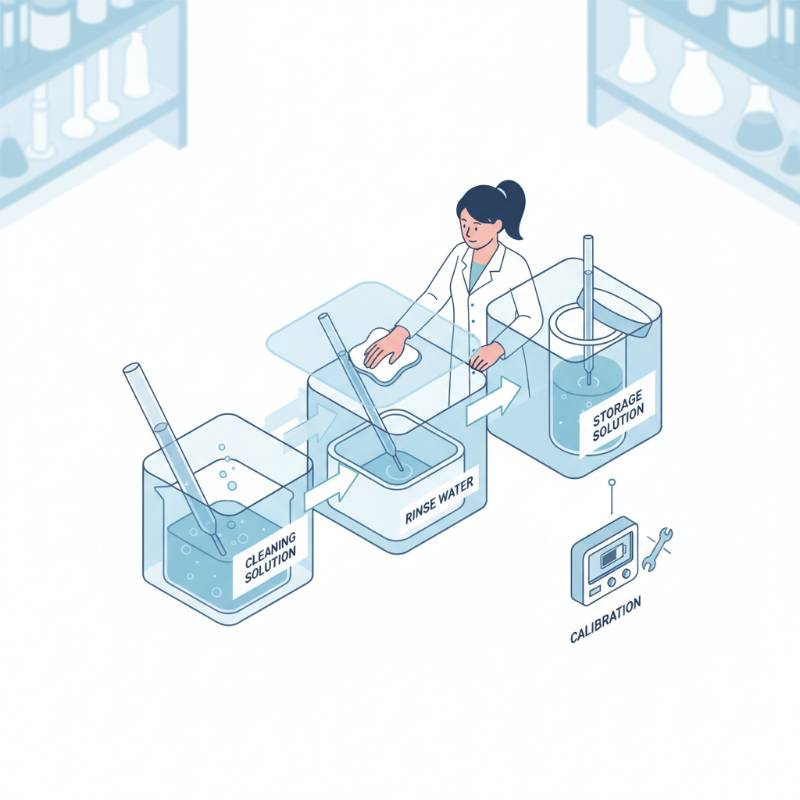
The 'Frit-First' Maintenance Protocol: Our Proprietary 4-Step Reliability Framework
Most researchers blame the silver wire when things go wrong. They're usually wrong. According to our analysis, 90% of reference electrode failures occur at the ceramic frit (the porous junction). We have developed The Frit-First Protocol to ensure long-term stability.
Step 1: The Clarity Check
Inspect the ceramic frit for discoloration. If it turns black or brown, metal ions from your analyte have likely precipitated inside the pores. This creates high resistance and "ghost peaks" in your data.
Step 2: Pressure Flushing
Gently apply pressure to the filling hole to force a few drops of electrolyte through the frit. This clears out microscopic blockages. If no liquid emerges, the junction is "blind" and needs replacing.
Step 3: Overnight Re-equilibration
Never use a dry electrode. If it has been out of solution, soak the frit in the internal filling solution for at least 12 hours before use. This restores the ionic pathway.
Step 4: Potential Verification
Compare your electrode against a "Master" reference kept only for testing. If the difference is >5mV, it is time to re-coat the Ag/AgCl wire or replace the unit.
Compatibility Matrix: Navigating Solvents and pH Ranges
Ag/AgCl electrodes are workhorses, but they have enemies. Using them in the wrong environment is a fast track to a "poisoned" electrode. If you are working with platinum-coated titanium anodes in extreme pH, you must be careful.
Aqueous Solutions: Perfect. This is where Ag/AgCl shines.
Strongly Alkaline (pH > 13): Risky. Silver oxides can form, shifting the potential. Consider a Hg/HgO electrode instead.
Sulfide/Cyanide Solutions: Dangerous. These ions react with AgCl to form highly insoluble silver salts, permanently clogging the junction.
Organic Solvents (Acetonitrile, DMF): Use a double-junction setup. KCl is often insoluble in organics and will precipitate, blocking the frit.
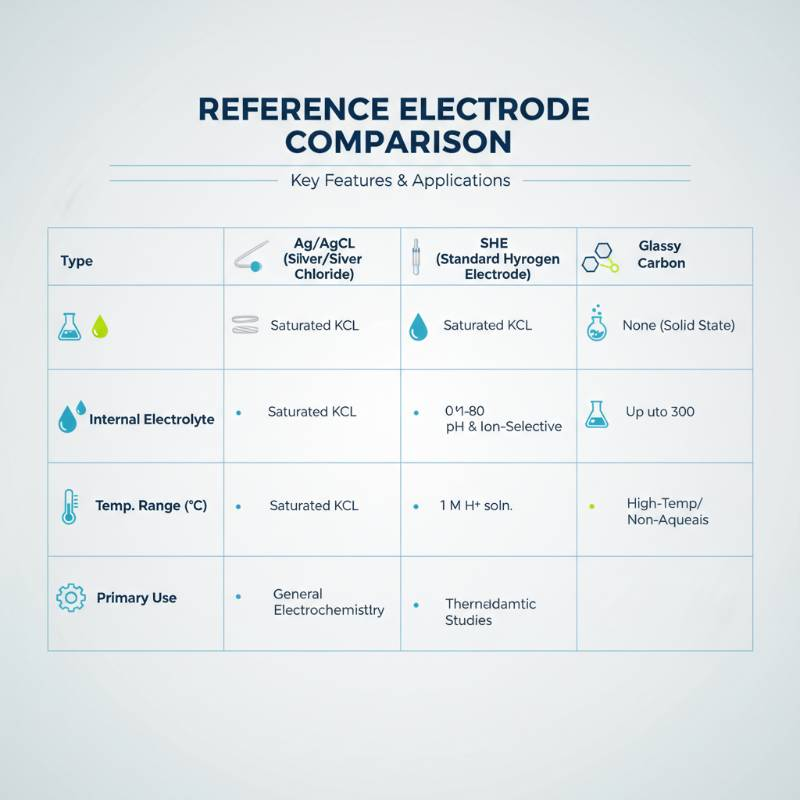
Ag/AgCl vs. The Rest: A Decision-Tree Comparison
Choosing the right reference is about matching the chemistry of your cell. While Ag/AgCl is the most common, it isn't always the best. For instance, when using MMO anodes in high-chloride seawater applications, the Ag/AgCl system is ideal because it mirrors the environment.
Use Ag/AgCl if: You need a robust, non-toxic, and low-maintenance option for neutral or acidic aqueous solutions.
Use SCE (Calomel) if: You require the highest possible precision and don't mind the environmental hazards of mercury.
Use Hg/HgO if: You are working in highly caustic, alkaline batteries or fuel cells.
Essential Maintenance: Keeping Your 'Sacrificial Lamb' Healthy
Don't treat your electrode like a piece of glassware. It is a living chemical system. To prevent potential drift, follow these hard rules:
Keep it topped off: Ensure the internal electrolyte level is always higher than the level of the sample solution. This prevents sample "backflow" into the electrode.
Kill the bubbles: A single air bubble trapped behind the ceramic frit can break the circuit. Flick the electrode like a thermometer to dislodge them.
Seal the hole: When not in use, close the filling hole to prevent evaporation and the formation of KCl crystals.
Frequently Asked Questions about Ag/AgCl Electrodes
Why is my reference potential drifting?
Drift is usually caused by temperature changes or a clogged junction. If the internal KCl concentration changes due to evaporation, the potential will shift. Check for salt crystals around the cap.
Can I use an Ag/AgCl electrode in non-aqueous solvents?
Yes, but you must use a bridge tube (double junction). Fill the outer chamber with a compatible electrolyte like TBAP in acetonitrile to prevent KCl from crashing out in your organic sample.
How do I store the electrode long-term?
Store it vertically in a solution identical to its internal filling solution (e.g., 3M KCl). Never store it in deionized water, as this will leach the ions out of the frit and destroy the equilibrium.
Ready for Precision-Engineered Electrodes?
Stop fighting with drifting baselines and unreliable data. Get the hardware that industry leaders trust for consistency.
Browse Our Technical Catalog